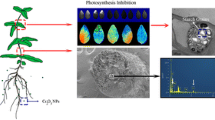
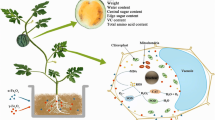
Abstract
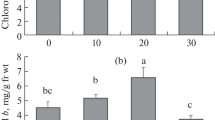
Nanoparticles (NPs) have been reported to cause physiological effects on plant cells and tissue. This study traced the uptake and distribution of magnetic iron oxide nanoparticles (γ-Fe2O3 NPs) in citrus (Citrus reticulata) plants under hydroponic condition by fluorescent dye labeled γ-Fe2O3 NPs, and described a detailed evidence of physiological effects of 0–100 mg/L γ-Fe2O3 NPs on citrus plants by measuring the physiological parameters such as content of chlorophyll, malondialdehyde (MDA), soluble sugar, soluble protein, activity of antioxidant enzyme, and ferric reductase after 21 days exposure. Fluorescence images of citrus stem and root showed that citrus roots could absorb γ-Fe2O3 NPs but no translocation from roots to shoots was observed, since NPs aggregated or even clogged the vascular system. Physiological results showed that 20 mg/L γ-Fe2O3 NPs could significantly enhance chlorophyll content by 126.4%, while 50 and 100 mg/L of γ-Fe2O3 NPs decreased chlorophyll content by 27.8 and 35.4%, respectively. MDA contents in citrus leaves under 20–100 mg/L γ-Fe2O3 NPs exposure were increased by 37.8, 107.2, and 61.5%, respectively, while that in roots were decreased by 27.0,11.9, and 7.4%, respectively, with elevated SOD and CAT activity, suggesting that oxidative stress occurred in citrus leaves, but oxidative stress in roots was eliminated by antioxidant defense. It is noteworthy that although Fe(II)-EDTA treatment had a high level of chlorophyll content, it induced strong oxidative stress in citrus plants as well. Collectively, the various physiological responses of citrus plants to γ-Fe2O3 NPs exposure were closely correlated with the concentrations of NPs. γ-Fe2O3 NPs at proper concentrations, such as 20 mg/L, have the potential to ameliorate chlorosis of plants and be effective nanofertilizers for increasing agronomic productivity.






Similar content being viewed by others
References
Abadía, J., Vázquez, S., Rellán-Álvarez, R., El-Jendoubi, H., Abadía, A., Álvarez-Fernández, A., et al. (2011). Towards a knowledge-based correction of iron chlorosis. Plant Physiology and Biochemistry, 49, 471–482.
Asli, S., & Neumann, P. M. (2009). Colloidal suspensions of clay or titanium dioxide nanoparticles can inhibit leaf growth and transpiration via physical effects on root water transport. Plant, Cell & Environment, 32, 577–584.
Bai, J., Liu, J., Zhang, N., & Sa, R. (2013). Effect of salt stress on antioxidant enzymes, soluble sugar and yield of oat. Advance Journal of Food Science & Technology, 5(3), 303–309.
Bienfait, H. F. (1985). Regulated redox processes at the plasmalemma of plant root cells and their function in iron uptake. Journal of Bioenergetics and Biomembranes, 17, 73–83.
Chen, J., Tian, Y., Zhang, X., Zheng, C., Song, Z., Deng, A., et al. (2014). Nighttime warming will increase winter wheat yield through improving plant development and grain growth in north China. Journal of Plant Growth Regulation, 33, 397–407.
Couée, I., Sulmon, C., Gouesbet, G., & Amrani, A. E. (2006). Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. Journal of Experimental Botany, 57(3), 449–59.
Gallego, S. M., Benavídes, M. P., & Tomaro, M. L. (1996). Effect of heavy metal ion excess on sunflower leaves: evidence for involvement of oxidative stress. Plant Science, 121, 151–159.
Ghafariyan, M. H., Malakouti, M. J., Dadpour, M. R., Stroeve, P., & Mahmoudi, M. (2013). Effects of magnetite nanoparticles on soybean chlorophyll. Environmental Science & Technology, 47, 10645–10652.
Han, Y., Fan, S., Zhang, Q., & Wang, Y. (2013). Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agricultural Sciences, 4(5), 112–115.
He, S., Feng, Y., Ren, H., Zhang, Y., Gu, N., & Lin, X. (2011). The impact of iron oxide magnetic nanoparticles on the soil bacterial community. Journal of Soils and Sediments, 11, 1408–1417.
Heath, R. L., & Packer, L. (1968). Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics, 125, 189–198.
Hong, Y., Honda, R. J., Myung, N. V., & Walker, S. L. (2009). Transport of iron-based nanoparticles: role of magnetic properties. Environmental Science & Technology, 43, 8834–8839.
Hong, J., Wang, L., Sun, Y., Zhao, L., Niu, G., Tan, W., et al. (2016). Foliar applied nanoscale and microscale CeO2 and CuO alter cucumber (Cucumis sativus) fruit quality. The Science of the Total Environment, 563, 904–911.
Kuboirie, M., Yokoyama, M., Shinkai, Y., Niki, R., Takeda, K., & Irie, M. (2016). The transfer of titanium dioxide nanoparticles from the host plant to butterfly larvae through a food chain. Scientific Reports, 6, 23819–23826.
Li, J., Chang, P. R., Huang, J., Wang, Y., Yuan, H., & Ren, H. (2013). Physiological effects of magnetic iron oxide nanoparticles towards watermelon. Journal of Nanoscience and Nanotechnology, 13, 1–7.
Li, J. L., Hu, J., Ma, C. X., Wang, Y. Q., Wu, C., Huang, J., et al. (2016). Uptake, translocation and physiological effects of magnetic iron oxide (γ-Fe2O3) nanoparticles in corn (Zea mays L.). Chemosphere, 159, 326–334.
Liu, R., Zhang, H., & Lal, R. (2016). Effects of stabilized nanoparticles of copper, zinc, manganese, and iron oxides in low concentrations on lettuce (Lactuca sativa) seed germination: nanotoxicants or nanonutrients? Water, Air, and Soil Pollution, 227, 1–14.
Ma, C., Chhikara, S., Xing, B. S., Musante, C., White, J., & Dhankher, O. P. (2013). Physiological and molecular response of Arabidopsis thaliana (L.) to nanoparticle cerium and indium oxide exposure. ACS Sustainable Chemistry & Engineering, 1, 768–778.
Ma, C., White, J. C., Dhankher, O. P., & Xing, B. S. (2015). Metal-based nanotoxicity and detoxification pathways in higher plants. Environmental Science & Technology, 49, 7109–7122.
Majumdar, S., Trujilloreyes, J., Hernandezviezcas, J. A., White, J. C., Peraltavidea, J. R., & Gardeatorresdey, J. L. (2016). Cerium biomagnification in a terrestrial food chain: influence of particle size and growth stage. Environmental Science & Technology, 50(13), 6782–6792.
Martínez-Fernández, D., Barroso, D., & Komárek, M. (2016). Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environmental Science and Pollution Research, 23, 1732–1741.
Means, J. L., Crerar, D. A., & Duguid, J. O. (1978). Migration of radioactive wastes: radionuclide mobilization by complexing agents. Science, 200, 1477–1481.
Nowack, B. (2002). Environmental chemistry of aminopolycarboxylate chelating agents. Environmental Science & Technology, 36, 4009–4016.
Peters, R. J., Bouwmeester, H., Gottardo, S., Amenta, V., Arena, M., Brandhoff, P., et al. (2016). Nanomaterials for products and application in agriculture, feed and food. Trends in Food Science and Technology, 54, 155–164.
Procter, C. M., Connolly, E. L., Robinson, N. J., & Guerinot, M. L. (1999). A ferric-chelate reductase for iron uptake from soils. Nature, 397, 694–697.
Reed, R., Ladner, D., Higgins, C. P., Westerhoff, P., & Ranville, J. (2012). Solubility of nanozinc oxide in environmentally and biologically important matrices. Environmental Toxicology and Chemistry, 31, 93–99.
Ren, H., Liu, L., Liu, C., He, S., Huang, J., Li, J., et al. (2011). Physiological investigation of magnetic iron oxide nanoparticles towards chinese mung bean. Journal of Biomedical Nanotechnology, 7, 677–684.
Rosa, M., Prado, C., Podazza, G., Interdonato, R., González, J. A., Hilal, M., et al. (2009). Soluble sugars—metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant Signaling & Behavior, 4(5), 388–393.
Servin, A. D., Morales, M. I., Castillomichel, H., Hernandezviezcas, J. A., Munoz, B., Zhao, L., et al. (2013). Synchrotron verification of TiO2 accumulation in cucumber fruit: a possible pathway of TiO2 nanoparticle transfer from soil into the food chain. Environmental Science & Technology, 11592-11598.
Singh, N. K., Bracken, P. M., Hasegawa, P. M., Handa, A. K., Buckel, S., Hermodson, M. A., et al. (1987). Characterization of osmotin A thaumatin-like protein associated with osmotic adjustment in plant cells. Plant Physiology, 85, 529–536.
Sjogren, C. E., Johansson, C., Naevestad, A., Sontum, P. C., Brileysaebo, K., & Fahlvik, A. K. (1997). Crystal size and properties of superparamagnetic iron oxide (SPIO) particles. Magnetic Resonance Imaging, 15, 55–67.
Song, U., Jun, H., Waldman, B., Roh, J., Kim, Y., Yi, J., et al. (2013). Functional analyses of nanoparticle toxicity: a comparative study of the effects of TiO2 and Ag on tomatoes (lycopersicon esculentum). Ecotoxicology and Environmental Safety, 93, 60–67.
Tan, X., & Fugetsu, B. (2007). Multi-walled carbon nanotubes interact with cultured rice cells: evidence of a self-defense response. Journal of Biomedical Nanotechnology, 3, 285–288.
Van-Bavel, C. H. M., Nakayama, F. S., & Ehrler, W. L. (1965). Measuring transpiration resistance of leaves. Plant Physiology, 40, 535–540.
Wang, Y. H., Ying, Y., Chen, J., & Wang, X. C. (2004). Transgenic arabidopsis overexpressing Mn-SOD enhanced salt-tolerance. Plant Science, 167, 671–677.
Wang, H., Kou, X., Pei, Z., Xiao, J. Q., Shan, X., & Xing, B. S. (2011). Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (lolium perenne L.) and pumpkin (cucurbita mixta) plants. Nanotoxicology, 5, 30–42.
Wu, C., Chang, P. R., Ren, H. X., Li, J. L., Huang, J., & Yuan, H. (2016). Fluorescent labelled iron oxide nanoparticles for tracing their uptake behavior by plant. Nano Reports, 2, 1–6.
Ylivainio, K. (2010). Effects of iron (III) chelates on the solubility of heavy metals in calcareous soils. Environmental Pollution, 158, 3194–3200.
Zhang, J., Cui, S., Li, J., & Kirkham, M. B. (1995). Protoplasmic factors, antoxidant responses, and chilling resistance in maize. Plant Physiology and Biochemistry, 33, 567–575.
Zhao, L., Sun, Y., Hernandez-Viezcas, J., Servin, A., Hong, J., Niu, G., et al. (2013). Influence of CeO2 and ZnO nanoparticles on cucumber physiological markers and bioaccumulation of Ce and Zn: a life cycle study. Journal of Agricultural and Food Chemistry, 61, 11945–11951.
Zhu, H., Han, J., Xiao, J. Q., & Jin, Y. (2008). Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. Journal of Environmental Monitoring, 10, 713–717.
Zuverza-Mena, N., Martínez-Fernández, D., Du, W., Hernandez-Viezcas, J. A., Bonilla-Bird, N., López-Moreno, M. L., et al. (2016). Exposure of engineered nanomaterials to plants: insights into the physiological and biochemical responses—a review. Plant Physiology and Biochemistry. doi:10.1016/j.plaphy.2016.05.037.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant no. 31301735), China Agriculture Research System (CARS-26-34), and the Fundamental Research Funds for the Central Universities (WUT:2016IB006); the International Science & Technology Cooperation Program, Science and Technology Department of Hubei Province (Grant No. 2016AHB028).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 14192 kb)
Rights and permissions
About this article
Cite this article
Li, J., Hu, J., Xiao, L. et al. Physiological Effects and Fluorescence Labeling of Magnetic Iron Oxide Nanoparticles on Citrus (Citrus reticulata) Seedlings. Water Air Soil Pollut 228, 52 (2017). https://doi.org/10.1007/s11270-016-3237-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-016-3237-9