Abstract
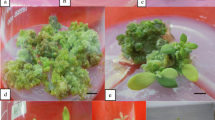
Black ash (Fraxinus nigra) is an endangered hardwood tree species under threat of extirpation by the emerald ash borer (EAB), an aggressive exotic phloem-feeding beetle. We have developed an efficient regeneration system through adventitious shoot organogenesis in F. nigra using in vitro-derived leaf explants. Two types of leaf explants were cultured on Murashige and Skoog (MS) medium supplemented with different concentrations of plant growth regulators to induce callus and adventitious shoot bud formation. Significant effects of explant, and plant growth regulator interactions were found. The frequency of callus formation ranged from 77.8 to 94.4% and 88.9–100% from single leaflets and intact compound leaves, respectively, with no significant difference between treatments. For adventitious shoot bud induction, however, 22.2 µM 6-benzylaminopurine (BA) combined with 31.8 µM thidiazuron (TDZ) was the best treatment regardless of the initial leaf explant type, showing 21.1 and 28.8% shoot bud induction, with 1.5 and 1.9 adventitious shoots per explant, from single leaflets and intact compound leaves, respectively. The regenerated shoot buds were elongated on MS medium supplemented with Gamborg B5 vitamins plus 2 mg L−1 glycine (MSB5G), 13.3 µM BA, 1 µM indole-3-butyric acid (IBA), and 0.29 µM gibberellic acid. The elongated shoots were continuously micropropagated through nodal stem sectioning until used for rooting. An average of 85.2% of the microshoots were successfully rooted in woody plant medium containing 5.7 µM indole-3-acetic acid plus 4.9 µM IBA with a 10-day initial dark culture, followed by culture under a 16-h photoperiod. Rooted plantlets were acclimatized to the greenhouse and showed normal plant growth and development with 100% survival. This regeneration protocol would be useful for mass propagation for conservation of F. nigra and for use in genetic transformation for EAB resistance.



Similar content being viewed by others
References
Anderson MK, Nesom G (2003) Black ash, Fraxinus nigra Marsh. NRCS Plant Guide. Natl Res Conserv Serv https://plants.usda.gov/plantguide/pdf/cs_frni.pdf
Bates S, Preece JE, Navarrete NE, Van Sambeek JW, Gaffney GR (1992) Thidiazuron stimulates shoot organogenesis and somatic embryogenesis in white ash (Fraxinus americana L.). Plant Cell Tiss Organ Cult 31:21–29
Beasley RR, Pijut PM (2013) Regeneration of plants from Fraxinus nigra Marsh. hypocotyls. HortScience 48:887–890
Benedict L, David R (2003) Propagation protocol for black ash (Fraxinus nigra Marsh.). Native Plants J 4:100–103
Benedict MA, Frelich LE (2008) Site factors affecting black ash ring growth in northern Minnesota. Forest Ecol Manag 255:3489–3493
Bergmann BA, Moon HK (1997) In vitro adventitious shoot production in Paulownia. Plant Cell Rep 16:315–319
Chalupa V (1988) Large scale micropropagation of Quercus robur L. using adenine-type cytokinins and thidiazuron to stimulate shoot proliferation. Biol Plantarum 30:414–421
Corredoira E, Ballester A, Vieitez AM (2008) Thidiazuron-induced high-frequency plant regeneration from leaf explants of Paulownia tomentosa mature trees. Plant Cell Tiss Organ Cult 95:197–208
Deore A, Johnson T (2008) High-frequency plant regeneration from leaf-disc cultures of Jatropha curcas L.: an important biodiesel plant. Plant Biotechnol Rep 2:7–11
Du N, Pijut PM (2008) Regeneration of plants from Fraxinus pennsylvanica hypocotyls and cotyledons. Sci Hortic 118:74–79
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gucker CL (2005) Fraxinus nigra. In: Fire Effects Information System. USDA, Forest Service, Washington DC http://www.fs.fed.us/database/feis/plants/tree/franig/all.html.
Guo B, Abbasi B, Zeb A, Xu L, Wei Y (2011) Thidiazuron: a multi-dimensional plant growth regulator. Afr J Biotechnol 10:8984–9000
Gupta S, Mahalaxmi V (2009) In vitro high frequency direct plant regeneration from whole leaves of blackberry. Sci Hortic 120:22–26
Harding K, Benson EE, Roubelakis-Angelakis KA (1996) Methylated DNA changes associated with the initiation and maintenance of Vitis vinifera in vitro shoot and callus cultures: a possible mechanism for age-related changes. Vitis 35:79–85
Herms DA, McCullough DG (2014) Emerald ash borer invasion of North America: history, biology, ecology, impacts, and management. Annu Rev Entomol 59:13–30
Huetteman C, Preece J (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tiss Organ Cult 33:105–119
IBM-SPSS (2015) SPSS version 23.0, IBM-SPSS, Armonk, NY
Iverson L, Knight KS, Prasad A, Herms DA, Matthews S, Peters M, Smith A, Hartzler DM, Long R, Almendinger J (2016) Potential species replacements for black ash (Fraxinus nigra) at the confluence of two threats: emerald ash borer and a changing climate. Ecosystems 19:248–270
Jin S, Wang J, Wang X, Sun D, Li G, Genovesi AD, Liu S (2014) Direct and indirect shoot and bulblet regeneration from cultured leaf explants of Lilium pumilum, an endangered species. In Vitro Cell Dev Biol Plant 50:69–75
Kahia J, Sallah PK, Diby L, Kouame C, Kirika M, Niyitegeka S, Asiimwe T (2015) A novel regeneration system for tamarillo (Cyphomandra betacea) via organogenesis from hypocotyl, leaf, and root explants. HortScience 50:1375–1378
Klooster WS, Herms DA, Knight KS, Herms C, McCullough DG, Smith A, Gandhi, KJK, Cardina J (2014) Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol Invasions 16:859–873
Kovacs KF, Haight RG, McCullough DG, Mercader RJ, Siegert NW, Liebhold AM (2010) Cost of potential emerald ash borer damage in U.S. communities, 2009–2019. Ecol Econ 69:569–578
Kumar PP, Dimps C, Goh CJ (1998) Influence of petiole and lamina on adventitious shoot initiation from leaf explants of Paulownia fortunei. Plant Cell Rep 17:886–890
Lloyd GB, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Proc Int Plant Prop Soc 30:421–427
Miguel CM, Druart P, Oliveira MM (1996) Shoot regeneration from adventitious buds induced on juvenile and adult almond (Prunus dulcis Mill.) explants. In Vitro Cell Dev Biol Plant 32:148–153
Mockeliunaite R, Kuusiene S (2004) Organogenesis of Fraxinus excelsior L. by isolated excelsior mature embryo culture. Acta Univ Latviensis Biol 676:197–676
Mohammed A, Chiruvella KK, Namsa ND, Ghanta RG (2015) An efficient in vitro shoot regeneration from leaf petiolar explants and ex vitro rooting of Bixa orellana L.—a dye yielding plant. Physiol Mol Biol Plants 21:417–424
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Murthy BNS, Murch SJ, Saxena PK (1995) Thidiazuron-induced somatic embryogenesis in intact seedlings of peanut (Arachis hypogaea): Endogenous growth regulator levels and significance of cotyledons. Physiol Plantarum 94:268–276
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potent regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol Plant 34:267
Nisbet D, Kreutzweiser D, Sibley P, Scarr T (2015) Ecological risks posed by emerald ash borer to riparian forest habitats: a review and problem formulation with management implications. Forest Ecol Manag 358:165–173
Palla KJ, Pijut PM (2011) Regeneration of plants from Fraxinus americana hypocotyls and cotyledons. In Vitro Cell Dev Biol Plant 47:250–256
Pérez-Tornero O, Egea J, Vanoostende A, Burgos L (2000) Assessment of factors affecting adventitious shoot regeneration from in vitro cultured leaves of apricot. Plant Sci 158:61–70
Poland TM, McCullough DG (2006) Emerald ash borer: invasion of the urban forest and the threat to North America’s ash resource. J Forestry 104:118–124
Rathore MS, Mastan SG, Yadav P, Bhatt VD, Shekhawat NS, Chikara J (2016) Shoot regeneration from leaf explants of Withania coagulans (Stocks) Dunal and genetic stability evaluation of regenerates with RAPD and ISSR markers. S Afr J Bot 102:12–17
Sarwar M, Skirvin RM (1997) Effect of thidiazuron and 6-benzylaminopurine on adventitious shoot regeneration from leaves of three strains of McIntosh apple (Malus x domestica Borkh.) in vitro. Sci Hortic 68:95–100
Singh C, Raj S, Patil V, Jaiswal P, Subhash N (2013) Plant regeneration from leaf explants of mature sandalwood (Santalum album L.) trees under in vitro conditions. In Vitro Cell Dev Biol Plant 49:216–222
Slazak B, Sliwinska E, Saługa M, Ronikier M, Bujak J, Słomka A, Göransson U, Kuta E (2015) Micropropagation of Viola uliginosa (Violaceae) for endangered species conservation and for somaclonal variation-enhanced cyclotide biosynthesis. Plant Cell Tiss Organ Cult 120:179–190
Stevens M, Pijut P (2012) Hypocotyl derived in vitro regeneration of pumpkin ash (Fraxinus profunda). Plant Cell Tiss Organ Cult 108:129–135
Subramaniam S, Rathinam X, Poobathy R, Sinniah U (2008) In vitro production of multiple bud clumps (Mbcs) from Cavendish banana cultivar, Brasilian (AAA). Am Eurasian J Sustain Agr 2:300–307
Tonon G, Capuana M, Di Marco A (2001) Plant regeneration of Fraxinus angustifolia by in vitro shoot organogenesis. Sci Hortic 87:291–301
Vanstone DE, LaCroix LJ (1975) Embryo immaturity and dormancy of black ash [Fraxinus nigra]. J Amer Soc Hort Sci 100:630–632
Vieitez AM, San-José MC (1996) Adventitious shoot regeneration from Fagus sylvatica leaf explants in vitro. In Vitro Cell Dev Biol Plant 32:140–147
Wang Y, Chen F, Wang Y, Li X, Liang H (2014) Efficient somatic embryogenesis and plant regeneration from immature embryos of Tapiscia sinensis Oliv., an endemic and endangered species in China. HortScience 49:1558–1562
Wright JK, Rauscher HM (1990) Fraxinus nigra Marsh., black ash. In: Burns RM, Honkala BH (eds) Silvics of North America: 2. Hardwoods, Agric Handbook. USDA Forest Services, Washington DC, p 688–693
Zaytseva YG, Poluboyarova TV, Novikova TI (2016) Effects of thidiazuron on in vitro morphogenic response of Rhododendron sichotense Pojark. and Rhododendron catawbiense cv. Grandiflorum leaf explants. In Vitro Cell Dev Biol Plant 52:56–63
Acknowledgements
This research was supported by partial funding from the USDA-APHIS-PPQ Center for Plant Health Science and Technology, the U.S. Endowment for Forestry and Communities, and members of the Indiana Hardwood Lumbermen’s Association. The authors gratefully acknowledge Drs. Armand Séguin and Jayasankar Subramanian for their constructive review and suggestions for the improvement of this manuscript, and Dale Simpson for the black ash seed. Mention of a trademark, proprietary product, or vendor does not constitute a guarantee or warranty of the product by the U.S. Department of Agriculture and does not imply its approval to the exclusion of other products or vendors that also may be suitable.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Angeles Revilla.
Rights and permissions
About this article
Cite this article
Lee, J.H., Pijut, P.M. Adventitious shoot regeneration from in vitro leaf explants of Fraxinus nigra . Plant Cell Tiss Organ Cult 130, 335–343 (2017). https://doi.org/10.1007/s11240-017-1228-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1228-1