Abstract
We established a red callus from the leaves of a red-fleshed apple individual, which was a hybrid offspring of the cross between Malus sieversii f. niedzwetzkyana and Malus domestica cv. ‘Fuji’. We analyzed callus growth and anthocyanin biosynthesis/metabolism under different combinations of temperature and light conditions. Incubation in darkness resulted in decreased anthocyanin accumulation, while it promoted callus growth. Exposure to light and low temperature (16 °C) induced the expression of MYB10 and bHLH3/33, which are responsible for coordinating the regulation of anthocyanin biosynthesis, as well as the expression of other structural genes. Treatments with light and high temperature (32 °C) induced MYB16 expression, which repressed anthocyanin biosynthesis. Additionally, low temperature (16 °C) inhibited the expression of MYB111. We analyzed the expression patterns of MYB and bHLH transcription factor genes by quantitative real-time polymerase chain reaction. Our data suggest that light-induced regulation of anthocyanin biosynthesis is primarily caused by altered MYB10 transcript levels, while temperature-induced regulation is the result of changes to the expression of bHLH3/33, MYB16, MYB17, MYB111, and other repressors. In conclusion, we investigated the reciprocal effects of light and temperature on anthocyanin biosynthesis in red-fleshed apple calli. Our findings may provide a theoretical basis for breeding red-fleshed apple varieties with high anthocyanin contents.
Similar content being viewed by others
Introduction
Anthocyanins are water-soluble natural pigments that provide color to various plant tissues and organs (Horbowicz et al. 2008). They also function as natural antioxidants that provide protection from free radicals and other harmful substances. Additionally, anthocyanins enhance vascular elasticity, prevent cardiovascular disease, and protect the liver from damage (Winkel-Shirley 2001; Regan et al. 2001; Schaefer et al. 2008; Butelli et al. 2008). In apple (Malus domestica Borkh.), the health benefits of flavonoids and anthocyanins have been investigated (Szankowski et al. 2009; Balasuriya and Rupasinghe 2012). The anthocyanin content in the pulp of most cultivated apples is very low and unstable (Nie et al. 2010). Malus sieversii f. niedzwetzkyana is a red-fleshed variant of M. sieversii. Its branches, leaves, flowers, fruit skin, and pulp are all red, with extremely high anthocyanin concentrations and considerable health benefits (Wang et al. 2010). Therefore, investigating the mechanism regulating anthocyanin biosynthesis in M. sieversii f. niedzwetzkyana is warranted, and may promote the breeding of red-fleshed apple varieties with high anthocyanin contents. Additional studies may also provide novel information regarding the genetics and diversity of cultivated apple species.
Anthocyanin biosynthesis is a process involving the coordinated expression of transcription factors (TFs) and structural genes (Dixon and Steele 1999; Takos et al. 2006). The MYB (ZmC1) and bHLH (ZmR and ZmB) TFs regulating anthocyanin biosynthesis were first detected in maize, and were subsequently isolated from petunia and Arabidopsis thaliana (Paz-Ares et al. 1988; Chandler et al. 1989; Quattrocchio et al. 1993; Borevitz et al. 2000). Investigations focused on anthocyanin biosynthesis in apple species occurred after these initial studies. The MdMYB1 and MdMYBA TFs were first isolated from apple skin, and were observed to regulate anthocyanin biosynthesis (Takos et al. 2006; Ban et al. 2007). The expression of MdMYB1 and MdMYBA is strongly induced by light. The MdMYB10 TF, which regulates color development in red apples, was isolated and identified from a red-fleshed apple cultivar (i.e., ‘Red Field’). Its regulatory activities require MdbHLH3 and MdbHLH33 (Espley et al. 2007).
In addition to genotype effects, environmental factors (e.g., light and temperature) play a crucial role in the accumulation of anthocyanins and endogenous hormones (Chandler et al. 1989; Quattrocchio et al. 1993; Borevitz et al. 2000; Solfanelli et al. 2006). In A. thaliana, the expression of MYB (PAP1 and PAP2) and bHLH (TT8, EGL3, and GL3) TF genes involved in anthocyanin biosynthesis is induced by light (Cominelli et al. 2008). In contrast, high temperature can inhibit the expression of EGL3, TTG1, and TT8, thus inhibiting the biosynthesis and accumulation of anthocyanins (Rowan et al. 2009). Low temperature and light intensity have a synergistic effect on the expression of genes in the flavonoid biosynthesis pathway in grape berry skin (Azuma et al. 2012). In apple fruit skin, high intensity light can induce the expression of MdMYB1 and promote the accumulation of anthocyanins (Takos et al. 2006). Low temperatures and UV-B irradiation can significantly increase the expression of related genes to promote the accumulation of anthocyanins in apple fruit skin (Ubi et al. 2006). Results of a previous study indicated that high temperatures inhibit MYB10 expression, resulting in a decrease in the biosynthesis and accumulation of anthocyanins (Lin-Wang et al. 2011).
To date, the light- and temperature-regulated mechanisms controlling anthocyanin biosynthesis have been partially characterized. However, because of variabilities in growth position, developmental phase, and nutritional status, it is difficult to accurately analyze the effects of environmental factors on anthocyanin biosynthesis in fruits. Furthermore, light, temperature, and other environmental conditions cannot be controlled during field experiments, and exactly which TFs are regulated by environmental conditions is currently unclear. Consequently, there have been few reports describing the relationship between light and temperature effects on anthocyanin accumulation in M. sieversii f. niedzwetzkyana red callus tissue. How structural genes and the MYB and bHLH TFs respond to various combinations of light and temperature to regulate anthocyanin biosynthesis has not been comprehensively investigated.
We used M. sieversii f. niedzwetzkyana germplasm available from the Luntai National Fruit Germplasm Resources Garden (Xinjiang Academy of Agricultural Science) as the parents to generate hybrids in 2006. Wang et al. (2010) studied a red-fleshed apple individual (‘Zihong 1’) obtained from segregating populations, and determined that its flesh contains extremely high anthocyanin contents. Ji et al. (2015) induced callus formation using a red-fleshed apple strain, and observed that increasing auxin concentrations can significantly inhibit anthocyanin biosynthesis. In this study, we generated a red callus using the leaves of ‘Zihong 1’ apple trees. The red callus responded to environmental changes. This callus tissue represented an uncommon material for studying the regulation of anthocyanin biosynthesis in red-fleshed apples. We investigated the effects of different temperature and light conditions on anthocyanin metabolism in red callus tissue, as well as the expression of related genes. Our objective was to generate novel molecular information regarding anthocyanin biosynthesis regulated by light and temperature conditions, which may be useful for the breeding of new red-fleshed apple cultivars.
Materials and methods
Plant materials and callus induction
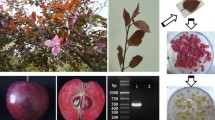
The F1 hybrid population of the cross between M. sieversii f. niedzwetzkyana and M. domestica cv. ‘Fuji’ was grown at the Tai’an Hengling Fruit Tree Breeding Base of Shandong Agricultural University (36° 260 N, 117° 290 E). We used young ‘Zihong 1’ leaves as explants for callus cultures (Fig. 1a). The red callus culturing method was based on a published procedure (Ji et al. 2015). The callus induction medium consisted of Murashige and Skoog medium supplemented with 0.6 mg l−1 2,4-dichlorophenoxyacetic acid, 0.5 mg l−1 thidiazuron, 30 g l−1 sugar, and 7 g l−1 agar. The pH was adjusted to 5.8 ± 0.1. Surface-sterilized leaves were first cultured at 24 °C for 15 days in darkness, and then cultured under light (16-h light/8-h dark; intensity: 1000–2000 lx). The red callus was finally induced and subcultured every 20 days (Fig. 1c).
Determination of callus growth
The callus/cell cultures (fresh weight: 0.15 g) were transferred to new culture flasks containing Murashige and Skoog medium. The calli were incubated under the following temperature and light conditions: 16 °C/light, 24 °C/light, 32 °C/light, 16 °C/dark (shade treatment), 24 °C/dark, and 32 °C/dark. Other growth parameters were the same for all cultures. The callus was harvested at 0, 5, 10, 15, and 20 days after the initiation of the cultures. Three culture flasks per treatment (i.e., biological replicates) were collected at each time point. The callus fresh weight was determined before samples were frozen in liquid nitrogen and stored at −80 °C until analyzed.
Measurement of relative anthocyanin contents
Callus samples frozen with liquid nitrogen were ground into a fine powder, and 0.5 g ground material was incubated in 15 ml 1 % (v/v) HCl-methanol for 24 h at 4 °C in darkness. Samples were centrifuged at 8000×g for 10 min, and the upper aqueous phase was subjected to spectrophotometric analysis at 530 nm using a UV-2450 spectrophotometer (Shimadzu, Kyoto, Japan). Relative anthocyanin contents were calculated as follows: absorbance (at 530 nm)/fresh weight (g).
HPLC analysis of anthocyanin extracts
Anthocyanin was extracted from 1 g powdered callus tissue. Samples were incubated in 5 ml 1 % (v/v) HCl-methanol for 2 h at 4 °C in darkness, and then centrifuged at 8000×g for 15 min. The upper aqueous phase was saved, and the pelleted material was incubated in 5 ml of 1 % (v/v) HCl-methanol for 1 h at 4 °C in darkness. Samples were centrifuged as before, and the two upper aqueous phases were combined and concentrated using a RE-52AA rotary evaporator (YaRong, Shanghai, China). Concentrated samples were rinsed in 2–3 ml methanol, transferred to 10-ml test tubes, and centrifuged at 8000×g for 20 min. The upper aqueous phase was diluted with methanol to 5 ml and filtered through an organic filter membrane (pore size: 0.2 µm). The HPLC analysis of anthocyanin extracts was conducted using an ACQUITY UPLC System (Waters Corporation, USA) with a BEH C18 chromatographic column (100 mm × 2.1 mm). The column particle size was 1.7 μm. Samples were eluted at a column temperature of 45 °C using a flow rate of 0.3 ml min−1. The mobile phases consisted of solvent A (acetonitrile) and solvent B [formic acid/water, 1:500 (v/v)] in the following gradient: 0–0.1 min, 5 % solvent A; 20 min, 20 % solvent A; 22 min, 80 % solvent A; 21 min, 5 % solvent A; and 25 min, 5 % solvent A. The HPLC eluates were monitored spectrophotometrically (530 nm).
Phylogenetic analysis and protein sequence alignment of transcription factors
A total of 24 MYB TFs from apple and other plant species (e.g., A. thaliana, Fragaria ananassa, Vitis vinifera, Zea mays, and Petunia hybrida) were used for phylogenetic analysis and protein sequence alignment. Half of the TFs were reported to promote anthocyanin biosynthesis, while the other half were known repressors of anthocyanin biosynthesis. Full-length TF protein sequences were obtained from the NCBI database, and aligned using DNAMAN software. Phylogenetic analyses were conducted using MEGA5.1 software with 1000 bootstrap replicates after aligning sequences with ClustalW (opening = 10, extension = 0.2).
RNA isolation and quantitative real-time polymerase chain reaction
Total RNA was isolated from callus tissues using an RNAprep Pure Plant kit (Tiangen, Beijing, China). The concentration and quality of the purified RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). First-strand cDNA was synthesized from 1 µg total RNA using the RevertAid First Strand cDNA Synthesis kit (Fermentas, Hanover, MD, USA). The cDNA samples were stored at −20 °C until used.
The quantitative real-time polymerase chain reaction (qRT-PCR) primers were designed using the Beacon Designer 7 program (Table S1). Primers were synthesized by Sangon Biotech (Shanghai, China) and purified by polyacrylamide gel electrophoresis. The qRT-PCR was conducted using tenfold diluted cDNA samples as templates, the SYBR Green PCR Master Mix (TransGen Biotech, Beijing, China), and the iCycler iQ5 system (Bio-Rad, Hercules, CA). The MdActin gene served as an internal control, and the relative quantities of mRNA were calculated using the 2−ΔΔCt method of the IQ5 2.0 program (Livak and Schmittgen 2001).
Results
Comparative analysis of callus growth under various conditions
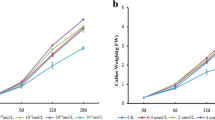
We observed significant differences in the growth of the red calli exposed to different light conditions and temperatures (Fig. 2). Callus growth was greatest at 24 °C/dark, followed by 24 °C/light, 32 °C/dark, 32 °C/light, 16 °C/dark, and 16 °C/light. The growth was inhibited at 32 and 16 °C, indicating that high and low temperatures can inhibit callus growth, although the inhibitory effect was particularly evident at the lower temperature. The fresh weights of calli incubated at 16 °C for 20 days were eightfold lower than the fresh weights of calli incubated at 24 °C. Additionally, we observed that at all temperatures, the fresh weight of calli grown in darkness was higher than that of calli incubated under light. This finding indicated that a lack of light was conducive to cell division and callus growth.
Comparative analysis and measurement of dynamic anthocyanin levels
To investigate the dynamic anthocyanin levels during the 20-day culture period, the absorbance (at 530 nm) of anthocyanin extracts was measured at different time points. We observed significant differences in the callus pigment and anthocyanin contents (Fig. 3). After a 20-day incubation under light, anthocyanins had considerably accumulated at 16 °C, and the calli appeared purple. As the temperature increased, the redness of the calli faded (Fig. 3a). All calli incubated in darkness were yellow. The relative anthocyanin contents over 20 days are presented in Fig. 3b. The anthocyanin contents continuously decreased in calli incubated in darkness. In contrast, in calli grown under light, the anthocyanin levels initially decreased, but then slowly increased. The callus anthocyanin levels on day 20 were highest at 16 °C/light, followed by 24 °C/light, 32 °C/light, 16 °C/dark, 24 °C/dark, and 32 °C/dark. These results suggest that under the same light condition, low (16 °C) and high (32 °C) temperatures can promote and inhibit anthocyanin biosynthesis, respectively. The anthocyanin content of calli incubated in darkness was very low, especially at 32 °C, and the relative content on day 20 was only 0.06, which was 27-fold lower than that of calli incubated under light.
Relative callus anthocyanin contents at different temperature and light conditions. a Changes in callus color under different conditions and culture stages. Bar 1 cm. b Relative anthocyanin contents over 20 days. The relative anthocyanin content was calculated as follows: absorbance (at 530 nm)/fresh weight (g)
A scatter diagram was generated to analyze the correlation between relative anthocyanin content and callus growth rate (Fig. 4). Higher callus anthocyanin contents tended to correspond with lower callus growth rates, suggesting there was a negative correlation between callus anthocyanin content and growth rate.
HPLC analysis of callus cyanidin 3-O-galactoside content
There is currently very little diversity regarding apple anthocyanins. Cyanidin 3-O-galactoside is the main apple anthocyanin, accounting for 80 % of the total anthocyanin content (Treutter 2001). Therefore, the callus cyanidin-3-galactoside contents were determined (Fig. 5a). According to our HPLC analysis, the retention time of cyanidin-3-galactoside was about 13 min (Fig. 5b). Figure 5c provides a close-up view of the region indicated by a red box in Fig. 5b. There was significant variability among the peak areas for the various treatments, with the largest differences observed for calli incubated at 16 °C/light (a), followed by calli grown at 24 °C/light (b), 32 °C/light (c), 16 °C/dark (d), 24 °C/dark (e), and 32 °C/dark (f). These results were consistent with the differences in anthocyanin contents. The absolute cyanidin-3-galactoside content was calculated by comparing the peak areas of treated calli with those of standard samples. We observed that calli incubated at 16 °C/light accumulated the most cyanidin-3-galactoside (up to 695.55 µg g−1). This abundance was 1.8-fold higher than that of calli cultured at 24 °C/light, and threefold higher than that of calli grown at 32 °C/light (Table 1).
HPLC chromatograms of anthocyanins from calli grown under different culture conditions. a Comparison of the colors of calli treated with different conditions for 20 days. b HPLC graph of the cyanidin-3-galactoside content of calli exposed to different treatments. c Close-up view of the region in the red box in (B), with (a)–(f) indicating the following treatments: (a) 16 °C/light, (b) 24 °C/light, (c) 32 °C/light, (d) 16 °C/dark, (e) 24 °C/dark, and (f) 32 °C/dark
Phylogenetic analysis and multiple sequence alignment of MYB transcription factors
Many studies have revealed that the anthocyanin biosynthesis pathway is affected by MYB TFs, which can function as inducers or repressors. In A. thaliana, MYB75 and MYB90 promote anthocyanin biosynthesis, and the expression of their genes is induced by light (Borevitz et al. 2000). In contrast, MYB3, MYB4, and MYB6 suppress anthocyanin accumulation (Zhou et al. 2015). These known A. thaliana MYBs were used as queries in BLAST searches of various plant genomes, including the apple genome, to identify MYB homologs (Fig. 6). The phylogenetic tree indicated that MdMYB10 and MdMYB1 are homologous to PyMYB10, VvMYBA1, and AtMYB75, which positively regulate anthocyanin biosynthesis. A multiple sequence alignment revealed that the anthocyanin biosynthesis-promoting TFs contain the R2R3 domain and bHLH motif (Fig. 6c). We also determined that MdMYB16, MdMYB17, and MdMYB111 are homologous to PyMYB6, VvMYB4, AtMYB3, and AtMYB4, which negatively regulate anthocyanin biosynthesis. According to a multiple sequence alignment, these TFs contain the subgroup 4 (pdLNLD/EL) suppression motif (Stracke et al. 2001) (Fig. 6d).
Phylogenetic analysis and multiple sequence alignment of MYB transcription factors from various plants. a Phylogenetic analysis of MYB transcription factors reported to promote anthocyanin biosynthesis. b Phylogenetic analysis of MYB transcription factors reported to inhibit anthocyanin biosynthesis. c Multiple sequence alignment of MYB transcription factors. All of the anthocyanin biosynthesis-promoting transcription factors contained the R2R3 domain and bHLH motif. d Multiple sequence alignment of transcription factors that negatively regulate anthocyanin biosynthesis. These transcription factors contained the subgroup 4 (pdLNL-D/EL) suppression motif
Expression levels of anthocyanin pathway genes and related transcription factors
We analyzed the expression levels of six structural genes from the anthocyanin biosynthesis pathway (i.e., CHI, CHS, F3H, LDOX, UFGT, and DFR) in calli exposed to different culture conditions for 20 days (Fig. 7). Under the same light conditions, all of these structural genes were most highly expression at 16 °C. The expression levels in calli incubated at 16 °C/light were 1.2–2-fold higher than in calli incubated at 24 °C. Additionally, the expression levels were 2–10-fold higher at 16 °C/light than at 32 °C. In contrast, the expression levels of structural genes (except CHI) were significantly inhibited at 32 °C. The CHS, F3H, LDOX, UFGT, and DFR expression levels at 32 °C were approximately 7-, 2-, 3-, 3-, and 2-fold lower than at 24 °C, and 11-, 3-, 5-, 5-, and 3-fold lower than at 16 °C, respectively. In calli incubated in darkness, the expression levels of all of the analyzed structural genes were relatively low.
Relative expression levels of the anthocyanin biosynthesis pathway structural genes under different culture conditions. CHS Chalcone synthase, CHI chalcone isomerase, F3H flavanone 3-hydroxylase, DFR dihydroflavonol 4-reductase, LDOX leucoanthocyanidin dioxygenase, UFGT UDP-flavanone-3-O-glucosyltransferase. MdActin was used as an internal control
To validate the regulatory mechanism of TFs during anthocyanin biosynthesis under different light and temperature conditions, the expression levels of MYB TF (MYB10, MYB16, MYB17, and MYB111) and bHLH TF (bHLH3 and bHLH33) genes were analyzed by qRT-PCR (Fig. 8). The MYB10 transcript levels were higher in calli incubated under light than in calli grown in darkness (i.e., approximately 17-, 6-, and 13-fold higher at 16, 24, and 32 °C, respectively). Additionally, temperature also affected the MYB10 expression levels under light, with 16 °C inducing the highest expression levels and 32 °C inhibiting expression. MYB16, MYB17, and MYB111 were speculated to be anthocyanin biosynthesis repressors. The highest MYB16 expression level was observed at 32 °C/light, and was about 2–3-fold higher than at other temperature and light conditions. MYB111 expression was inhibited by low temperature under light and dark conditions, which promoted the accumulation of anthocyanins. The MYB17 expression levels were higher in calli incubated in darkness, with 32 °C inducing the highest expression levels. BHLH is another important TF family involved in regulating anthocyanin biosynthesis. The bHLH3 and bHLH33 expression levels were highest at 16 °C, and were approximately twofold higher than at other temperatures (Fig. 8).
Discussion
Effect of light and temperature on callus growth and anthocyanin biosynthesis
Apple is one of the most widely cultivated fruit trees in the world, with many cultivars producing fruits with a red peel (Chen et al. 2010). Fruit color is an important apple trait, and largely determines the market value of apple crops. Anthocyanins are the main pigments affecting the color and nutritional value of apple fruits, and there has been considerable interest in their synthesis and regulation among apple researchers (Takos et al. 2006; Ubi et al. 2006; Espley et al. 2007; Lin-Wang et al. 2011). As a type of antioxidant, anthocyanins have important implications for human health (Smith et al. 2000; Wang and Mazza 2002; Chun et al. 2004). However, anthocyanins accumulate only in the red peel of cultivated apple fruits, with very little (or none at all) detected in the white fruit flesh (Nie et al. 2010). Most studies on anthocyanins have concentrated on the apple peel, which is not the main part of consumers to eat. Meng et al. (2016) studied anthocyanin biosynthesis in red-skinned cultivars at different fruit development stages, and suggested that the expression of the responsible genes is induced by light. Wang et al. (2006) observed that anthocyanin contents of apple peels increase significantly following exposure to continuous light. Other researchers determined that UV-B irradiation can induce anthocyanin biosynthesis in apple peels, and the regulatory effect of UV-B is enhanced at low temperatures (Ubi et al. 2006).
In this study, we developed a red callus culture system using red-fleshed apple ‘Zihong1’. The generated calli exhibited consistent growth and development, making them appropriate for studies on anthocyanin biosynthesis regulated by light, temperature, and other environmental factors. We observed that incubations in darkness significantly inhibited the accumulation of anthocyanins. The anthocyanin content of calli grown in darkness was only 4–10 % of that of calli exposed to light. Low temperatures are conducive to anthocyanin biosynthesis. The anthocyanin content was significantly higher at 16 °C than at 24 or 32 °C. These findings are consistent with the results of previous studies, indicating that temperature and light regulate anthocyanin biosynthesis not only in red-skinned cultivated apples, but also in red-fleshed apples and red callus tissue. Thus, anthocyanin metabolism may be relatively stable in response to different temperatures and light conditions, and is not influenced by different genotypes or tissues.
Interestingly, Ji et al. (2015) studied anthocyanin accumulation in the same red callus at different concentration of auxins and/or cytokinins, and suggested that anthocyanin accumulation decreased with increased auxin (NAA and 2,4-D) concentration, while nitrogen deficiency could reverse the inhibition of anthocyanin synthesis by auxins. Both growth regulators and nitrogen affected the primary metabolism of the plant. Growth accumulation and secondary metabolite require different conditions to induce a shift from the growth state to the metabolite production state (Simões et al. 2012). Our results confirmed this statement, and found that callus growth is influenced by temperature and light treatments, and that callus growth and anthocyanin content are negatively correlated to a certain extent. Therefore, there may be a competitive relationship between the growth state and the metabolite production state in plant. This is consistent with the apple fruit developmental process in which the fruit first increases in size during the primary growth stage, and then secondary metabolic activities lead to the accumulation of anthocyanins.
Regulatory effects of light and temperature on related transcription factors and structural genes
Light and temperature can induce the expression of related TF and structural genes to regulate anthocyanin metabolism. In apple skin, CHS, ANS, F3H, DFR, and UFGT have been isolated and identified (Honda et al. 2002). Among these genes, ANS and UFGT have important functions during anthocyanin biosynthesis. Their expression is induced by UV-B irradiation and low temperatures in apple fruit skin (Kondo et al. 2002; Kim et al. 2006). In in vitro culture systems, some studies have suggested that light irradiation can induce anthocyanin production in Catharanthus roseus (Hall and Yeoman 1986), Centaurea cyanus (Kakegawa et al. 1987), Perilla frutescens (Zhong et al. 1993), Strawberry (Sato et al. 1996) and other plant species. In contrast, the effect of temperature on anthocyanin synthesis was more variable. The highest anthocyanin accumulation was at 30 °C when compared to lower temperatures in callus cultures of Daucus carota (Narayan et al. 2005), while low temperature enhanced both anthocyanin accumulation and CHS expression in petunia flower (Shvarts et al. 1997). In this study, we focused on the expression of TF and structural genes in calli treated with different combinations of temperatures and light conditions. The ANS, F3H, CHS, DFR, and UFGT expression patterns based on our data are consistent with those of previous studies. The expression level of the five genes was higher under light than in darkness, and increasing temperatures resulted in decreasing expression levels. We observed that the expression of CHI was induced by low temperature. Additionally, its expression was not inhibited by high temperature, unlike the expression of CHS and F3H. The expression levels of LDOX, DFR, and UFGT decreased with increasing temperature.
A previous study on TFs concluded that light is an essential factor influencing the color of apple fruit skin (Takos et al. 2006). Li et al. confirmed that MdMYB1 protein stability is enhanced by exposure to light, leading to increased anthocyanin biosynthesis in apple skin (Li et al. 2012). Azuma et al. (2012) reported that low temperature and light have a synergistic effect on the expression of the flavonoid biosynthesis pathway genes in grape berry skin. Tian et al. (2015) found that low temperatures induced the expression of McMYB10, McbHLH3/33 and McTTG1, leading to anthocyanin accumulation in crabapple leaves. In this study, we observed that MdMYB10 expression was up-regulated in calli cultured under light, while the expression was very low in calli grown in darkness, which is in agreement with earlier findings regarding anthocyanin biosynthesis. Furthermore, we revealed that temperature can also regulate MdMYB10 expression, as indicated by the fact its expression level decreased with increasing temperatures.
The bHLH TFs and the negative regulatory factors, MYB16, MYB17, and MYB111, were unaffected by light, but they were sensitive to temperature. The mechanism underlying the regulation of anthocyanin biosynthesis by temperature is relatively complex. Low temperatures increase the expression level of anthocyanin biosynthesis genes in Z. mays, V. vinifera, Citrus sinensis, and other plants (Christie et al. 1994; Lo Piero et al. 2005; Mori et al. 2005). Additionally, MdbHLH3 promotes anthocyanin accumulation and fruit coloration in apples at low temperatures (Xie et al. 2012). In this study, bHLH3 and bHLH33 were highly expressed in calli grown at 16 °C/light, implying that the expression of bHLH TF genes may be induced by low temperature, but not light, to regulate anthocyanin accumulation. Heating apple fruits rapidly decreases the expression levels of MYB10, which is responsible for coordinating the development of red fruit skin color (Lin-Wang et al. 2011). We observed that high temperatures inhibited MYB10 expression. Additionally, some transcription repressors regulating anthocyanin biosynthesis were also affected by temperature. For example, MYB16 was highly expressed at 32 °C/light, indicating that high temperatures up-regulate MYB16 expression, leading to inhibited accumulation of anthocyanins. However, this regulatory mechanism did not apply to calli incubated in darkness. In contrast, the high temperature-induced expression of MYB17, and subsequent inhibition of anthocyanin accumulation, occurred in calli grown in darkness. MYB111 expression was repressed by low temperature in calli incubated under light and in darkness, which promoted anthocyanin accumulation. Based on these results, we considered the effects of different combinations of temperatures and light conditions on the mechanism regulating anthocyanin biosynthesis. We developed a model summarizing our findings regarding the effects of light and temperature on anthocyanin biosynthesis in apple (Fig. 9).
Conclusion
The results presented herein indicate that light is the main environmental factor influencing anthocyanin biosynthesis. It affects the accumulation of anthocyanins by regulating the expression of MYB10. Temperature is a secondary factor that helps regulate anthocyanin biosynthesis by mediating the production of bHLH TFs, MYB16, MYB17, and other repressors.
References
Azuma A, Yakushiji H, Koshita Y, Kobayashi S (2012) Flavonoid biosynthesis-related genes in grape skin are differentially regulated by temperature and light conditions. Planta 236(4):1067–1080
Balasuriya N, Rupasinghe HPV (2012) Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem 135(4):2320–2325
Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T (2007) Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin. Plant Cell Physiol 48(7):958–970
Borevitz JO, Xia Y, Blount J, Dixon RA, Lamb C (2000) Activation tagging identifies a conserved MYB regulator of phenylpropanoid biosynthesis. Plant Cell 12(12):2383–2393
Butelli E, Titta L, Giorgio M, Mock HP, Matros A, Peterek S, Wijlen EG, Hall RD, Bovy AG, Luo J (2008) Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat Biotechnol 26(11):1301–1308
Chandler VL, Radicella JP, Robbins TP, Chen J, Turks D (1989) Two regulatory genes of the maize anthocyanin pathway are homologous: isolation of B utilizing R genomic sequences. Plant Cell 1(12):1175–1183
Chen XS, Han MY, Su GL, Liu FZ, Guo GN, Jiang YM (2010) Discussion on today’s world apple industry trends and the suggestions on sustainable and efficient development of apple industry in China. J Fruit Sci 27:598–604
Christie PJ, Alfenito MR, Walbot V (1994) Impact of low-temperature stress on general phenylpropanoid and anthocyanin pathways: enhancement of transcript abundance and anthocyanin pigmentation in maize seedlings. Planta 194(4):541–549
Chun OK, Kim DO, Chang YL (2004) Superoxide radical scavenging activity of the major polyphenols in fresh plums. J Agric Food Chem 51(27):8067–8072
Cominelli E, Gusmaroli G, Allegra D, Galbiati M, Wade HK, Jenkins GI, Tonelli C (2008) Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J Plant Physiol 165(8):886–894
Dixon RA, Steele CL (1999) Flavonoids and isoflavonoids—a gold mine for metabolic engineering. Trends Plant Sci 4(10):394–400
Espley R, Hellens R, Putterill J, Stevenson DA, Allan AC (2007) Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. Plant J 49(3):414–427
Hall RD, Yeoman MM (1986) Factors determining anthocyanin yield in cell cultures of Catharanthus roseus (L.) G. Don. New Phytol 103:33–43
Honda C, Kotoda N, Wada M, Kondo S, Kobayashi S, Soejima J, Zhang Z, Tsuda T, Moriguchi T (2002) Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol Biochem 40(11):955–962
Horbowicz M, Kosson R, Grzesiuk A, Debski H (2008) Anthocyanins of fruits and vegetables—their occurrence, analysis and role in human nutrition. Veg Crops Res Bull 68(68):5–22
Ji XH, Wang YT, Zhang R, Wu SJ, An MM, Li M, Wang CZ, Chen XL, Zhang YM, Chen XS (2015) Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell, Tissue Organ Cult 120(1):325–337
Kakegawa K, Kaneko Y, Hattori E, Koike K, Takeda K (1987) Cell cultures of Centaurea cyanus produces malonated anthocyanin in UV light. Phytochemistry 26:2261
Kim SH, Lee JR, Kim SR (2006) Characterization of an apple anthocyanidin synthase gene in transgenic tobacco plants. J Plant Biol 49(4):326–330
Kondo S, Hiraoka K, Kobayashi S, Honda C, Terahara N (2002) Changes in the expression of anthocyanin biosynthetic genes during apple development. J Am Soc Hortic Sci Am Soc Hortic Sci 127(6):971–976
Li YY, Mao K, Zhao C, Zhao XY, Zhang HL, Shu HR, Hao YJ (2012) MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple 1, [W] [OA]. Plant Physiol 160(2):1011–1022
Lin-Wang K, Micheletti D, Palmer J, Volz R, Lozano L, Espley R, Hellens RP, Chagne D, Rowan DD, Troggio M, Iglesias I, Allen AC (2011) High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex. Plant, Cell Environ 34(7):1176–1190
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 method. Methods 25(4):402–408
Lo Piero AR, Ivana P, Paolo R, Goffredo P (2005) Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J Agric Food Chem 53(23):9083–9088
Meng R, Zhang J, An L, Zhang B, Jiang X, Yang Y, Zhao Z (2016) Expression profiling of several gene families involved in anthocyanin biosynthesis in apple (Malus domestica. Borkh.) Skin during fruit development. J Plant Growth Regul 35(2):449–464
Mori K, Sugaya S, Gemma H (2005) Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci Hortic 105(3):319–330
Narayan MS, Thimmaraju R, Bhagyalakshmi B (2005) Interplay of growth regulators during solid-state and liquid-state batch cultivation of anthocyanin producing cell line of Daucus carota. Process Biochem 40:351–358
Nie JL, Lv DG, Li J, Liu FZ, Li HF, Xu GF, Wu YL (2010) Studies on composition and content of flavonoids in fruit of ‘Nagafu 2’ apple. Acta Hortic Sin 37(37):1559–1566
Paz-Ares J, Ghosal D, Wienand U, Peterson PA, Saedler H (1988) The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J 6(12):3553–3558
Quattrocchio F, Wing JF, Leppen H, Mol J, Koes RE (1993) Regulatory genes controlling anthocyanin pigmentation are functionally conserved among plant species and have distinct sets of target genes. Plant Cell 5(11):1497–1512
Regan BC, Julliot C, Simmen B, Viénot F, Charles-Dominique P, Mollon JD (2001) Fruits, foliage and the evolution of primate colour vision. Philos Trans R Soc B Biol Sci 356(1407):229–283
Rowan DD, Mingshu C, Kui LW, Cooney JM, Jensen DJ, Austin PT, Hunt MB, Cara N, Hellens RP, Schaffer RJ (2009) Environmental regulation of leaf colour in red 35S:PAP1 Arabidopsis thaliana. New Phytol 182(1):102–115
Sato K, Nakayama M, Shigeta JI (1996) Culturing conditions affecting the production of anthocyanin in suspended cell cultures of strawberry. Plant Sci 113:91–98
Schaefer HM, McGraw K, Catoni C (2008) Birds use fruit colour as honest signal of dietary antioxidant rewards. Funct Ecol 22(2):303–310
Shvarts M, Borochov A, Weiss D (1997) Low temperature enhances petunia flower pigmentation and induces chalcone synthase gene expression. Physiol Plant 99(1):67–72
Simões C, Albarello N, Castro TCD, Mansur E (2012) Production of anthocyanins by plant cell and tissue culture strategies. Biotechnol Prod Plant Second Metab (5):67–86
Smith MAL, Marley KA, Seigler D, Singletary KW, Meline B (2000) Bioactive properties of wild blueberry fruits. J Food Sci 65(2):352–356
Solfanelli C, Bartolini S, Vitagliano C, Lorenzi R (2006) Immunolocalization and quantification of IAA after self- and free-pollination in Olea europaea L. Sci Hortic 110(4):345–351
Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Curr Opin Plant Biol 4(5):447–456
Szankowski I, Li H, Flachowsky H, Höfer M, Hanke MV, Fischer T, Forkmann G, Treutter D, Schwab W, Hoffmann T (2009) Metabolic engineering of flavonoid biosynthesis in apple (Malus domestica Borkh.). In: Proceedings of the 12th Eucarpia symposium on fruit breeding and genetics, Zaragoza, Spain, pp 511–516
Takos AM, Jaffé FW, Jacob SR, Jochen B, Robinson SP, Walker AR (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples & nbsp. Plant Physiol 142(3):1216–1232
Tian J, Han ZY, Zhang LR, Song TT, Zhang J, Li JY, Yao YC (2015) Induction of anthocyanin accumulation in crabapple (Malus cv.) leaves by low temperatures. Hortsci Publ Am Soc Hortic Sci 50(5):640–649
Treutter D (2001) Biosynthesis of phenolic compounds and its regulation in apple. Plant Growth Regul 34(1):71–89
Ubi B, Honda C, Bessho H, Kondo S, Wada M, Kobayashi ST (2006) Expression analysis of anthocyanin biosynthetic genes in apple skin: effect of UV-B and temperature. Plant Sci 170(3):571–578
Wang J, Mazza G (2002) Inhibitory effects of anthocyanins and other phenolic compounds on nitric oxide production in LPS/IFN-gamma-activated RAW 264.7 macrophages. J Agric Food Chem 50(4):850–857
Wang ZH, Tang GH, Li ZQ, Wang LJ (2006) Promotion of 5-am inolevulinic acid and genistein on anthocyanin accumulationin apples. Acta Hortic Sin 33:1055–1058
Wang Y, Chen XS, Liu DL, Wang CZ, Song Y, Chen XL, Zhang YM (2010) Antioxidant activity and anthocyanins analysis of pulp in ‘Zihong 1’ red-flesh apple. Acta Hortic Sin 39:1991–1998
Winkel-Shirley B (2001) Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126(2):485–493
Xie XB, Shen LI, Zhang RF, Zhao J, Chen YC, Zhao Q, Yao YX, You CX, Zhang XS, Hao YJ (2012) The bHLH transcription factor MdbHLH3 promotes anthocyanin accumulation and fruit colouration in response to low temperature in apples. Plant, Cell Environ 35(11):1884–1897
Zhong JJ, Yoshida M, Fujiyama K, Seki T, Yoshida T (1993) Enhancement of anthocyanin production by Perilla frutescens cells in a stirred bioreactor with internal light irradiation. J Ferment Bioeng 75(4):299–303
Zhou M, Sun Z, Wang C, Zhang X, Tang Y, Zhu X, Shao J, Wu Y (2015) Changing a conserved amino acid in R2R3-MYB transcription repressors results in their cytoplasmic accumulation and abolishes their repressive activity in Arabidopsis. Plant J 84(2):395–403
Acknowledgments
We thank the Luntai National Fruit Germplasm Resources Garden for providing the germplasm. We thank Shujing Wu for critically reading the manuscript. We appreciate the help of Yanting Wang, Min Li, Pingping Lv, Haifeng Xu, Shouqian Feng, and Xiaoliu Chen in setting up the experiments.
Authors contribution
Conceived and designed the experiments: NW ZYZ XSC. Performed the experiments: NW. Analyzed the data: NW ZYZ. Contributed to the writing of the manuscript: NW ZYZ XSC.
Author information
Authors and Affiliations
Corresponding author
Additional information
Nan Wang and Zongying Zhang have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wang, N., Zhang, Z., Jiang, S. et al. Synergistic effects of light and temperature on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell Tiss Organ Cult 127, 217–227 (2016). https://doi.org/10.1007/s11240-016-1044-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-1044-z