Abstract
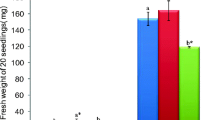
The effect of optimal and supra-optimal concentrations (0, 200, 400 or 600 mM) of NaCl on the growth, osmotic adjustment and antioxidant enzyme defence was studied in the in vitro cultures of Sesuvium portulacastrum. A significant increase in growth, tissue water content (TWC) and fresh to dry weight ratio (FW/DW) was observed in the shoots exposed to 200 mM salt. Minimum damage to the membrane in terms of low relative electrolytic leakage (REL) and malondialdehyde (MDA) content and better osmotic adjustment at 200 mM salt stress was coupled with the higher accumulation of sodium ions and total soluble sugars as against low proline and glycine betaine contents. A fine tuning of antioxidant enzyme activities (superoxide dismutase, catalase and ascorbate peroxidase) was also found to be responsible for the optimum growth of shoots. In contrast, sub-optimal (0 mM) and supra-optimal concentrations (400–600 mM) of NaCl significantly affected the growth, water status and increased the REL as well as MDA content of the shoots due to the accumulation of toxic concentrations of saline ions. The highest accumulation of proline and glycine betaine in addition to antioxidant enzyme activities exhibited higher osmotic adjustment and survival of the shoots under sub- or supra-optimal concentrations of NaCl as a penalty to reduced growth.






Similar content being viewed by others
Abbreviations
- APX:
-
Ascorbate peroxidase
- BA:
-
6-Benzyladenine
- CAT:
-
Catalase
- MDA:
-
Malondialdehyde
- MS:
-
Murashige and Skoog basal medium
- REL:
-
Relative electrolytic leakage
- SOD:
-
Superoxide dismutase
- TBA:
-
Thiobarbituric acid
- TCA:
-
Trichloroacetic acid
- TSS:
-
Total soluble sugars
- TWC:
-
Tissue water content
References
Balonkin YV, Kotov AA, Myasoedov NA, Khailova GF, Kurkova EB, Lun’kov RV, Kotova LM (2005) Involvement of long-distance Na+ transport in maintaining water potential gradient in the medium-root-leaf system of a halophyte Suaeda altissima. Russ J Plant Physiol 52:489–496
Basu S, Gangopadhyay G, Mukherjee BB (2002) Salt tolerance in rice in vitro: Implication of accumulation of Na+, K+ and proline. Plant Cell Tissue Organ Cult 69:55–64
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Becana M, Moran JF, Iturbe-Ormaetxe I (1998) Iron-dependent oxygen free radical generation in plants subjected to environmental stress: toxicity and antioxidant protection. Plant Soil 201:137–147
Bennici A, Corrado T (2009) Ultrastructural effects of salinity in Nicotiana bigelovii var. bigelovii callus cells and Allium cepa roots. Caryolog 62(2):124–133
Bracci T, Minnocci A, Sebastiani L (2008) In vitro olive (Olea europaea L.) cvs Frantoio and Moraiolo microshoot tolerance to NaCl. Plant Biosyst 142:563–571
Britto DT, Kronzucker HJ (2006) Futile cycling at the plasma membrane: a hallmark of low-affinity nutrient transport. Trends Plant Sci 11:529–534
Cakmak I, Marschner H (1992) Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol 98:1222–1227
Cano EA, Pérez-Alfocea F, Moreno V, Caro M, Bolarin MC (1998) Evaluation of salt tolerance in cultivated and wild tomato species through in vitro shoot apex culture. Plant Cell Tissue Organ Cult 53:19–26
Cherian S, Reddy MP (2003) Evaluation of NaCl tolerance in the callus cultures of Suaeda nudiflora Moq. Biol Plant 46:193–198
Daniells IG, Holland JF, Young RR, Alston CL, Bernardi AL (2001) Relationship between yield of grain sorghum (Sorghum bicolor) and soil salinity under field conditions. Aust J Plant Exp Agric 41:211–217
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Errabii T, Gandonou CB, Essalmani H, Abrini J, Idaomor M, Senhaji NS (2007) Effects of NaCl and mannitol induced stress on sugarcane (Saccharum sp.) callus cultures. Acta Physiol Plant 29:95–102
Flowers TJ, Colmer TD (2008) Salinity tolerance in halophytes. New Phytol 179:945–963
Flowers TJ, Hajibagheri MA, Clipson NJW (1986) Halophytes. Q Rev Biol 61:313–337
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Humphries EC (1956) Mineral components and ash analysis. In: Peach K, Tracey NV (eds) Modern methods of plant analysis, vol 1. Springer, Berlin, pp 468–502
James JJ, Alder NN, Mühling KH, Läuchli AE, Shackel KA, Donovan LA, Richards JH (2006) High apoplastic solute concentrations in leaves alter water relations of the halophytic shrub, Sarcobatus vermiculatus. J Expt Bot 57:139–147
Jithesh MN, Prashanth SR, Sivaprakash KR, Parida AK (2006) Antioxidative response mechanisms in halophytes: their role in stress defence. J Genet 85(3):237–254
Khan MH, Panda SK (2007) Alterations in root lipid peroxidation and antioxidative responses in two rice cultivars under NaCl-salinity stress. Acta Physiol Plant 30:81–89
Khan MA, Ungar IA, Showalter AM (2000) The effect of salinity on the growth, water status, and ion content of a leaf succulent perennial halophyte, Suaeda fruticosa (L.) Forssk. J Arid Environ 45:73–84
Lokhande VH, Nikam TD, Patade VY, Suprasanna P (2009a) Morphological and molecular diversity analysis among the Indian clones of Sesuvium portulacastrum L. Genet Resour Crop Evol 56:705–717
Lokhande VH, Nikam TD, Suprasanna P (2009b) Sesuvium portulacastrum (L.) L. a promising halophyte: cultivation, utilization and distribution in India. Genet Resour Crop Evol 56:741–747
Lokhande VH, Nikam TD, Suprasanna P (2010) Biochemical, physiological and growth changes in response to salinity in callus cultures of Sesuvium portulacastrum L. Plant Cell Tissue Organ Cult 102:17–25. doi: 10.1007/s11240-010-9699-3
Lovelock CE, Ball MC (2002) Influence of salinity on photosynthesis of halophytes. In: Läuchli A, Lüttge U (eds) Salinity: environment—plant—molecules. Kluwer, Dordrecht, pp 315–339
Messedi D, Labidi N, Grignon C, Abdelly C (2004) Limits imposed by salt to the growth of the halophyte Sesuvium portulacastrum. J Plant Nutr Soil Sci 167:720–725
Mills D, Zhang G, Benzioni A (2001) Effect of different salts and of ABA on growth and mineral uptake in Jojoba shoots grown in vitro. J Plant Physiol 158:1031–1039
Moseki B, Buru JC (2010) Ionic and water relations of Sesuvium portulacastrum (L). Sci Res Essay 5:35–40
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Ann Rev Plant Biol 59:651–681
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Parida AK, Das AB, Mohanty P (2004) Defense potentials to NaCl in a mangrove, Bruguiera parviflora: differential changes of isoforms of some antioxidative enzymes. J Plant Physiol 161:531–542
Pérez-Tornero O, Tallón CI, Porras I, Navarro JM (2009) Physiological and growth changes in micropropagated Citrus macrophylla explants due to salinity. J Plant Physiol 166(17):1923–1933
Raven JA (1985) Regulation of pH and generation of osmolarity in vascular land plants: costs and benefits in relation to efficiency of use of water, energy and nitrogen. New Phytol 101:25–77
Shigeoka S, Ishikawa T, Tamoi M, Miyagawa Y, Takeda T, Yabuta Y, Yoshimura K (2002) Regulation and function of ascorbate peroxidase isoenzymes. J Exp Bot 53:1305–1319
Šiler B, Mišić D, Filipović B, Popović Z, Cvetić T, Mijović A (2007) Effects of salinity on in vitro growth and photosynthesis of common centaury (Centaurium erythraea Rafn.). Arch Biol Sci Belgrade 59(2):129–134
Slama I, Ghnaya T, Savouré A, Abdelly C (2008) Combined effects of long-term salinity and soil drying on growth, water relations, nutrient status and proline accumulation of Sesuvium portulacastrum. C R Biol 331:442–451
Tester M, Davenport R (2003) Na+ tolerance and Na+ transport in higher plants. Ann Bot 91:503–527
Touchette BW (2006) Salt tolerance in a Juncus roemerianus brackish marsh: spatial variations in plant water relations. J Exp Marine Biol Ecol 337:1–12
Troncoso A, Matte C, Cantos M, Lavee S (1999) Evaluation of salt tolerance of in vitro-grown grapevine rootstock varieties. Vitis 38:55–60
Vicente O, Boscaiu M, Naranjo MA, Estrelles E, Bellés JM, Soriano P (2004) Responses to salt stress in the halophyte Plantago crassifolia (Plantaginaceae). J Arid Environ 58:463–481
Vijayan K, Chakraborti SP, Ghosh PD (2003) In vitro screening of mulberry (Morus spp.) for salinity tolerance. Plant Cell Rep 22:350–357
Watanabe S, Kojima K, Ide Y, Sasaki S (2000) Effects of saline and osmotic stress on proline and sugar accumulation in Populus euphratica in vitro. Plant Cell Tissue Organ Cult 63:199–206
Woodward AJ, Bennett IJ (2005) The effect of salt stress and abscisic acid on proline production, chlorophyll content and growth of in vitro propagated shoots of Eucalyptus camaldulensis. Plant Cell Tissue Organ Cult 82:189–200
Yang J, Yen HE (2002) Early salt stress effects on the changes in chemical composition in leaves of ice plant and Arabidopsis. A Fourier transform infrared spectroscopy study. Plant Physiol 130:1032–1042
Zhang F, Yang YL, He WL, Zhao X, Zhang LX (2004) Effects of salinity on growth and compatible solutes of callus induced from Populus euphratica. In Vitro Cell Dev Biol Plant 40:491–494
Zhu J-K (2001) Plant salt tolerance. Trends Plant Sci 6:66–71
Acknowledgements
The senior author is grateful to the Department of Atomic Energy (DAE), Board for Research in Nuclear Science (BRNS), for the financial support under the BARC-UOP collaborative Ph.D. research programme.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lokhande, V.H., Nikam, T.D., Patade, V.Y. et al. Effects of optimal and supra-optimal salinity stress on antioxidative defence, osmolytes and in vitro growth responses in Sesuvium portulacastrum L.. Plant Cell Tiss Organ Cult 104, 41–49 (2011). https://doi.org/10.1007/s11240-010-9802-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9802-9