Abstract
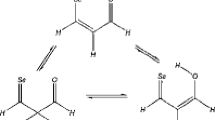
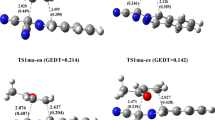
Due to the insufficient understanding of the intramolecular proton-transfer reactions in the heteroatoms-substituted propylene and pentadiene, relevant geometrical, electronic, and energetic properties of propylene derivatives (1–12) and pentadiene derivatives (13–24) have been investigated theoretically. It appears that, the “enol” form is thermodynamically less stable than the corresponding tautomer except for 9, 12, and 21. Owing to the stabilization effects of the heteroatoms, the proton-transfer reactions are more favorable for the heteroatoms-substituted cases than that of the corresponding propylene or pentadiene. The proton-transfer barriers in the propylene derivatives are higher than those in the corresponding pentadiene derivatives due to the different steric conditions for the formation of transition states. These barriers have a linear correlation with the charges of their transferred hydrogen atoms. Relevant geometric and electronic variations have also been studied. Specially, H2O assists the proton-transfer reactions in propylene derivatives, but inhibits these reactions in pentadiene derivatives. The above trends are slightly changed when the polarizable-continuum-based aqueous phase is considered.




Similar content being viewed by others
References
Bountis T (ed) (1992) Proton transfer in hydrogen-bonded systems. Plenum Press, New York
Scheiner S (1994) Acc Chem Res 27:402–408
Fonseca TAO, Freitas MP, Cormanich RA, Ramalho TC, Tormena CF, Rittner R (2012) Beilstein J Org Chem 8:112–117
Hess BA Jr, Schaad LJ (1983) J Am Chem Soc 105:7185–7186
Roth WR, König J (1966) Justus Liebigs Ann Chem 699:24–32
Bouma WJ, Poppinger D, Radom L (1977) J Am Chem Soc 99:6443–6444
Smith BJ, Nguyen MT, Bouma WJ, Radom L (1991) J Am Chem Soc 113:6452–6458
Juerschik S, Sulzer P, Petersson F, Mayhew CA, Jordan A, Agarwal B, Haidacher S, Seehauser H, Becker K, Maerk TD (2010) Anal Bioanal Chem 398:2813–2820
Hudson CE, McAdoo DJ (2003) J Org Chem 68:2735–2740
Gu Q, Trindle C, Knee JL (2012) J Chem Phys 137:091101/1–091101/4
Lammertsma K, Prasad BV (1994) J Am Chem Soc 116:642–650
Nagaoka M, Suenobu K, Yamabe T (1997) J Am Chem Soc 119:8023–8030
Raissi H, Farzad F, Eslamdoost S, Mollania F (2013) J Theor Comput Chem 12:1350025/1–1350025/18
Ladell J, Post B (1954) Acta Crystallogr 7:559–564
Leszczynski J, Kwiatkowski JS, Leszczynska D (1992) J Am Chem Soc 114:10089–10091
Leszczynski J, Kwiatkowski JS, Leszczynska D (1993) J Am Chem Soc 115:5891
Dapprich S, Frenking G (1993) Chem Phys Lett 205:337–342
Jensen F (1995) J Am Chem Soc 117:7487–7492
Tietze LF, Schulz G (1997) Chem Eur J 3:523–529
Dinadayalane TC, Geetha K, Sastry GN (2003) J Phys Chem A 107:5479–5487
Bosch E, Moreno M, Lluch JM (1992) J Am Chem Soc 114:2072–2076
Bach RD, Canepa C (1996) J Org Chem 61:6346–6353
Paul BK, Guchhait N (2011) Comput Theor Chem 972:1–13
González L, Mó O, Yáñez M (1997) J Phys Chem A 101:9710–9719
Nguyen MT, Weringa WD, Ha TK (1989) J Phys Chem 93:7956–7960
Lin JF, Wu CC, Lien MH (1995) J Phys Chem 99:16903–16908
Saettel NJ, Wiest O (2000) J Org Chem 65:2331–2336
Lavigueur C, Foster EJ, Williams VE (2008) J Am Chem Soc 130:11791–11800
Del BJE, Alkorta I, Sanchez-Sanz G, Elguero J (2012) J Phys Chem A 116:9205–9213
Yang X, Fox T, Berke H (2012) Org Biomol Chem 10:852–860
Basak A, Gupta SN, Chakrabarty K, Das GK (2013) Comput Theor Chem 1007:15–30
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, FarkasO, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson BG, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA Gaussian 03, Revision D.01, Gaussian, Wallingford, CT, 2004
Hay PJ, Wadt WR (1985) J Chem Phys 82:270–283
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3093
Miertuš S, Scrocco E, Tomasi J (1981) Chem Phys 55:117–129
Craw JS, Bacskay GB (1992) Faraday Trans J Chem Soc 88:2315–2321
Zhong A, Ge C, Liang H, Jiang H, Zhou Q (2012) Comput Theor Chem 988:13–18
Wilson LY, Famini GR (1991) J Med Chem 34:1668–1674
Fukui K (1971) Acc Chem Res 4:57–64
Markova N, Enchev V (2004) J Mol Struct (Theochem) 679:195–205
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, H., Li, H. Theoretical studies on the proton-transfer reactions in propylene and pentadiene derivatives. Struct Chem 26, 587–597 (2015). https://doi.org/10.1007/s11224-014-0521-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0521-4