Abstract
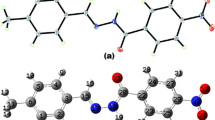
Theoretical study of several para-substituted N-nitrosoacetanilide biological molecules has been performed using density functional B3LYP method with 6-31G(d,p) basis set. Geometries obtained from DFT calculation were used to perform natural bond orbital analysis. The p characters of two nitrogen natural hybrid orbital (NHO) σ N3–N2 bond orbitals increase with increasing σ p values of the para substituent group on the benzene, which results in a lengthening of the N3–N2 bond. The p characters of oxygen NHO σ O1–N2 and nitrogen NHO σ O1–N2 bond orbitals decrease with increasing σ p values of the para substituent group on the benzene, which results in a shortening of the N2=O1 bond. It is also noted that decreased occupancy of the localized σ N3–N2 orbital in the idealized Lewis structure, or increased occupancy of \( \sigma_{\rm N3-N2}^{\ast}\) of the non-Lewis orbital, and their subsequent impact on molecular stability and geometry (bond lengths) are also related with the resulting p character of the corresponding nitrogen NHO of σ N3–N2 bond orbital.

Similar content being viewed by others
References
Bulter AR, Williams DLH (1993) Chem Soc Rev 22:233
Richter-Addo GB, Legzdins P (2002) J Chem Rev 102:857
Cheng J-P, Wang W, Yin Z, Zhu X-Q, Lu Y (1988) Tetrahedron Lett 39:7925
Kopylova BV, Kandror II, Freidlina RKh (1979) Russ Chem Bull 28:1064
Brydon DL, Cadogan JIG, Cook J, Harger MJP, Sharp JT (1971) J Chem Soc B 2475
Barclay LRC, Dust JM (1982) Can J Chem 60:607
Iglesias E (1998) J Am Chem Soc 120:13057
Ryzhov V, Klippenstein SJ, Dunbar RC (1996) J Am Chem Soc 118:5462
Li X–H, Tang Z-X, Zhang X–Z (2009) J Mol Struct 899:42
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head Gordon M, Replogle ES, Pople JA (2003) GAUSSIAN 03, Revision B.02, Gaussian Inc., Pittsburgh, PA
Becke AD (1993) J Chem Phys 98:5648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785
Glendening ED, Reed AE, Carpenter JE, Weinhold F (2003) NBO version 3.1, Gaussian Inc., Pittsburg, PA
Reed AE, Weinhold F (1985) J Chem Phys 83:1736
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735
Reed AE, Weinhold F (1983) J Chem Phys 78:4066
Foster JP, Weinhold F (1980) J Am Chem Soc 102:7211
Chocholousova J, Vladimir Spirko V, Hobza P (2004) Phys Chem Chem Phys 6:37
Cao CZ (2003) Substituent effects in organic chemistry. Science Press, Beijing
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899
Luo YR (2003) Handbook of bond dissociation energies in organic compounds. CRC Press, Boca Raton
Haser M, Ahlrichs R (1989) J Comput Chem 10:104
Ahlriches R, Barr M, Haser M, Horn H, Komel C (1989) Chem Phys Lett 162:65
Acknowledgment
We thank the National Natural Science Foundation of China (No. 10774039) for the generous support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, XH., Zhang, RZ. & Zhang, XZ. Natural bond orbital analysis of some para-substituted N-nitrosoacetanilide biological molecules. Struct Chem 20, 1049–1054 (2009). https://doi.org/10.1007/s11224-009-9508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9508-y