Abstract
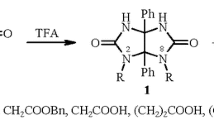
The α-ureidoalkylation of N-(hydroxyalkyl)ureas (ureido alcohols) with 1,3-H2- and 1,3-Me2-4,5-dihydroxyimidazolidin-2-ones was systematically studied. The yields of glycolurils decrease both in going from 1,3-H2- to 1,3-Me2-4,5-dihydroxyimidazolidin-2-one and with increasing length (or with branching) of the hydroxyalkyl chain in ureido alcohols. The optimal reaction time for ureido alcohols is 1 h. The X-ray diffraction study showed that 2-(2-hydroxy-1,1-dimethylethyl)glycoluril crystallizes as a conglomerate. The enantiomeric analysis of 2-(2-hydroxyethyl)glycoluril was carried out by chiral-phase HPLC.
Similar content being viewed by others
References
A. N. Kravchenko, K. A. Lyssenko, I. E. Chikunov, P. A. Belyakov, M. M. Ilyin, V. V. Baranov, Yu. V. Nelyubina, V. A. Davankov, T. S. Pivina, N. N. Makhova, and M. Yu. Anpitin, Izv. Akad. Nauk, Ser. Khim., 2009, 390 [Russ. Chem. Bull., Int. Ed., 2009, 58, No. 395].
M. D. Mashkovskii, Lekarstvennye sredstva [Drugs], Novaya volna, Moscow, 2005, 1, 86 (in Russian).
O. V. Lebedev, L. I. Khmel’nitskii, L. V. Epishina, L. I. Suvorova, I. V. Zaikonnikova, I. E. Zimakova, S. V. Kirshin, A. M. Karpov, V. S. Chudnovskii, M. V. Povstyanoi, V. A. Eres’ko, Tselenapravlennyi poisk novykh neirotropnykh preparatov [Targeted Search for New Neurotrophic Drugs], Zinatne, Riga, 1983, p. 81 (in Russian).
Yu. B. Vikharev, L. V. Anikina, I. E. Chikunov, A. S. Sigachev, A. N. Kravchenko, Yu. V. Shklyaev, N. N. Makhova, Vopr. Biol. Med. Farm. Khim. [Probl. Biol. Med Pharm. Chem.], 2006, 12 (in Russian).
K. A. Lyssenko, D. G. Golovanov, A. N. Kravchenko, I. E. Chikunov, O. V. Lebedev, N. N. Makhova, Mendeleev Commun., 2004, 105.
K. Yu. Chegaev, A. N. Kravchenko, O. V. Lebedev, Yu. A. Strelenko, Mendeleev Commun., 2001, 32.
A. N. Kravchenko, E. Yu. Maksareva, P. A. Belyakov, A. S. Sigachev, K. Yu. Chegaev, K. A. Lyssenko, O. V. Lebedev, N. N. Makhova, Izv. Akad. Nauk, Ser. Khim., 2003, 180 [Russ. Chem. Bull., Int. Ed., 2003, 52, 192].
G. A. Gazieva, D. G. Golovanov, P. V. Lozhkin, K. A. Lyssenko, A. N. Kravchenko, Zh. Neorg. Khim., 2007, 52, 1539 [Russ. J. Inorg. Chem. (Engl. Transl.), 2007, 52, 1441].
V. Z. Pletnev, I. Yu. Mikhailova, A. N. Sobolev, N. M. Galitskii, A. I. Verenich, L. I. Khmel’nitskii, O. V. Lebedev, A. N. Kravchenko, L. I. Suvorova, Bioorg. Khim., 1993, 19, 671 [Bioorgan. Chem. (Engl. Transl.), 1993, 19].
R. G. Kostyanovsky, G. K. Kadorkina, K. A. Lyssenko, V. Yu. Torbeev, A. N. Kravchenko, O. V. Lebedev, G. V. Grintselev-Knyazev, V. R. Kostyanovsky, Mendeleev Commun., 2002, 6.
I. Lahav, M. Leiserowitz, Crystal Growth Design, 2003, 3, 125.
Weygand-Hilgetag, Organisch-Chemische Experimentierkunst, Johann Ambrosius Barth, Vertag, Leipzig, 1964.
S. Vail, R. Barker, P. Mennitt, J. Org. Chem., 1965, 30, 2179.
H. Petersen, Lieb. Ann. Chem., 1969, 726, 89.
SHELXTL v. 5.10, Structure Determination Software Suite, Bruker AXS, Madison, Wisconsin, USA, 1998.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician O. N. Chupakhin on the occasion of his 75th birthday.
For Part 1, see Ref. 1.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 6, pp. 1229–1233, June, 2009.
Rights and permissions
About this article
Cite this article
Kravchenko, A.N., Sigachev, A.S., Belyakov, P.A. et al. 4,5-Dihydroxyimidazolidin-2-ones in α-ureidoalkylation of N-carboxy-, N-hydroxy-, and N-aminoalkylureas 2. α-Ureidoalkylation of N-(hydroxyalkyl)ureas. Russ Chem Bull 58, 1264–1269 (2009). https://doi.org/10.1007/s11172-009-0165-5
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-009-0165-5