Abstract
Purpose
To derive a health state classification system (HSCS) from the cancer-specific quality of life questionnaire, the EORTC QLQ-C30, as the basis for a multi-attribute utility instrument.
Methods
The conceptual model for the HSCS was based on the established domain structure of the QLQ-C30. Several criteria were considered to select a subset of dimensions and items for the HSCS. Expert opinion and patient input informed a priori selection of key dimensions. Psychometric criteria were assessed via secondary analysis of a pooled dataset comprising HRQOL and clinical data from 2616 patients from eight countries and a range of primary cancer sites, disease stages, and treatments. We used confirmatory factor analysis (CFA) to assess the conceptual model’s robustness and generalisability. We assessed item floor effects (>75 % observations at lowest score), disordered item response thresholds, coverage of the latent variable and differential item function using Rasch analysis. We calculated effect sizes for known group comparisons based on disease stage and responsiveness to change. Seventy-nine cancer patients assessed the relative importance of items within dimensions.
Results
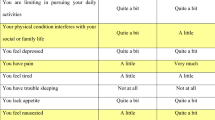
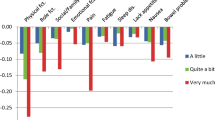
CFA supported the conceptual model and its generalisability across primary cancer sites. After considering all criteria, 12 items were selected representing 10 dimensions: physical functioning (mobility), role functioning, social functioning, emotional functioning, pain, fatigue, sleep, appetite, nausea, bowel problems.
Conclusions
The HSCS created from QLQ-C30 items is known as the EORTC Quality of Life Utility Measure-Core 10 dimensions (QLU-C10D). The next phase of the QLU-C10D’s development involves valuation studies, currently planned or being conducted across the globe.
Similar content being viewed by others
References
Aaronson, N. K., Bullinger, M., & Ahmedzai, S. (1988). A modular approach to quality of life assessment in cancer clinical trials. Recent Results in Cancer Research, 111, 231–249.
Aaronson, N. K., Ahmedzai, S., Bergman, B., Bullinger, M., Cull, A., Duez, N. J., et al. (1993). The European Organisation for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85(5), 365–376.
Fayers, P. M., Aaronson, N. K., Bjordal, K., Groenvold, M., Curran, D., Bottomley, A., on behalf of the EORTC Quality of Life Group. (2001). The EORTC QLQ-C30 scoring manual (3rd ed.). Brussels: European Organisation for Research and Treatment of Cancer (EORTC).
Drummond, M. F., Sculpher, M. J., Torrance, G. W., O’Brien, B. J., & Stoddart, G. L. (2005). Methods for the economic evaluation of health care programmes (3rd ed.). Oxford: Oxford University Press.
National Institute for Health and Clinical Excellence. (2013). Guide to the methods of technology appraisal. London: NICE.
Mittmann, N., Evans, W. K., Rocchi, A., Longo, C. J., Au, H.-J., Husereau, D., et al. (2009). Addendum to CADTH’s guidelines for the economic evaluation of health technologies: Specific guidance for oncology products. Ottawa: Canadian Agency for Drugs and Technologies in Health.
Mitchell, A. S., & Viney, R. (2010). Meeting the information needs of a national drug payer: Aspirations of the guidelines from Australia. Drug Development Research, 71(8), 463–469.
Scuffham, P. A., Whitty, J. A., Mitchell, A., & Viney, R. (2008). The use of QALY weights for QALY calculations: A review of industry submissions requesting listing on the Australian Pharmaceutical Benefits Scheme 2002–4. Pharmacoeconomics, 26(4), 297–310.
Neumann, P. J., Goldie, S. J., & Weinstein, M. C. (2000). Preference-based measures in economic evaluation in health care. Annual Review of Public Health, 21, 587–611.
Brazier, J., Roberts, J., & Deverill, M. T. (2002). The estimation of a preference-based measure of health from the SF-36. Journal of Health Economics, 21(2), 271–292.
Viney, R., Norman, R., King, M. T., Cronin, P., Street, D. J., Knox, S., & Ratcliffe, J. (2014). Time trade-off derived EQ-5D weights for Australia. Value in Health, 14(6), 928–936.
Norman, R., Viney, R., Brazier, J., Burgess, L., Cronin, P., King, M., et al. (2014). Valuing SF-6D health states using a discrete choice experiment. Medical Decision Making, 34(6), 773–786.
Viney, R., Norman, R., Brazier, J., Cronin, P., King, M. T., Ratcliffe, J., & Street, D. (2014). An Australian discrete choice experiment to value EQ-5D health states. Health Economics, 23(6), 729–742.
Brazier, J., Czoski-Murray, C., Roberts, J., Brown, M., Symonds, T., & Kelleher, C. (2008). Estimation of a preference-based index from a condition-specific measure: The King’s Health Questionnaire. Medical Decision Making, 28(1), 113–126.
Brazier, J., Usherwood, T., Harper, R., & Thomas, K. (1998). Deriving a preference-based single index from the UK SF-36 Health Survey. Journal of Clinical Epidemiology, 51(11), 1115–1128.
Young, T. A., Yang, Y., Brazier, J. E., & Tsuchiya, A. (2011). The use of Rasch analysis in reducing a large condition-specific instrument for preference valuation: The case of moving from AQLQ to AQL-5D. Medical Decision Making, 31(1), 195–210.
Young, T. A., Yang, Y., Brazier, J. E., Tsuchiya, A., & Coyne, K. (2009). The first stage of developing preference-based measures: Constructing a health-state classification using Rasch analysis. Quality of Life Research, 18(2), 253–265.
Norman, R., Viney, R., Aaronson, N. K., Brazier, J. E., Cella, D. F., Costa, D. S. J., et al. (2015). Using a discrete choice experiment to value the QLU-C10D: Feasibility and sensitivity to presentation format. Quality of Life Research. doi:10.1007/s11136-015-1115-3
Pickard, A. S., Shaw, J. W., Lin, H. W., Trask, P. C., Aaronson, N., Lee, T. A., & Cella, D. (2009). A patient-based utility measure of health for clinical trials of cancer therapy based on the European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire. Value in Health, 12(6), 977–988.
Bjordal, K., de Graef, A., Fayers, P. M., Hammerlid, E., van Pottelsberghe, C., Curran, D., et al. (2000). A 12 country field study of the EORTC QLQ-C30 (version 3.0) and the head and neck cancer specific module (EORTC QLQ-H&N35) in head and neck patients. European Journal of Cancer, 36(14), 1796–1807.
Costa, D. S. J., Aaronson, N. K., Fayers, P. M., Grimison, P., Janda, M., Pallant, J. F., et al. (2014). Deriving a preference-based utility measure for cancer patients from the EORTC QLQ-C30: A confirmatory versus exploratory approach. Patient Related Outcome Measures, 5, 119–129.
Costa, D. S. J., Aaronson, N. K., Fayers, P. M., Pallant, J. F., Velikova, G., & King, M. T. (2015). Testing the measurement invariance of the EORTC QLQ-C30 across primary cancer sites using multi-group confirmatory factor analysis. Quality of Life Research, 24(1), 125–133.
Pallant, J. F., & Tennant, A. (2007). An introduction to the Rasch measurement model: An example using the Hospital Anxiety and Depression Scale (HADS). British Journal of Clinical Psychology, 46, 1–18.
Revicki, D., Hays, R. D., Cella, D., Sloan, J., Revicki, D., Hays, R. D., et al. (2008). Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. Journal of Clinical Epidemiology, 61(2), 102–109.
Kazis, L. E., Anderson, J. J., & Meenan, R. F. (1989). Effect sizes for interpreting changes in health status. Medical Care, 27(3 Suppl), S178–S189.
Costa, D. S. J., King, M. T., Aaronson, N. K., Brazier, J. E., Cella, D., Grimison, P., et al. (2014). Do cancer patients distinguish between the importance and severity of their quality of life concerns? Quality of Life Research, 23(1 Supplement), 122.
Arraras, J. I., Suárez, J., Arias de la Vega, F., Vera, R., Asín, G., Arrazubi, V., et al. (2011). The EORTC Quality of Life questionnaire for patients with colorectal cancer: EORTC QLQ-CR29 validation study for Spanish patients. Clinical and Translational Oncology, 13(1), 50–56.
Arraras, J. I., Tejedor, M., Illaramendi, J. J., Vera, R., Pruja, E., Marcos, M., et al. (2001). El cuestionario de calidad de vida para cáncer de mama de la EORTC, QLQ-BR23: Estudio psicométrico con una muestra española. Psicologia Conductual, 9, 81–97.
Arraras Urdaniz, J. I., Villafranca Iturre, E., Arias de la Vega, F., Domínguez Domínguez, M. A., Lainez Milagro, N., Manterola Burgaleta, A., et al. (2008). The EORTC Quality of Life Questionnaire QLQ-C30 (version 3.0). Validation study for Spanish prostate cancer patients. Archivos Españoles de Urología, 61(8), 949–954.
Cengiz, M., Ozyar, E., Esassolak, M., Altun, M., Akmansu, M., Sen, M., et al. (2005). Assessment of quality of life of nasopharyngeal carcinoma patients with EORTC QLQ-C30 and H&N-35 modules. International Journal of Radiation Oncology Biology Physics, 63(5), 1347–1353.
Chie, W., Chang, K., Huang, C., & Kuo, W. (2003). Quality of life of breast cancer patients in Taiwan: Validation of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-BR23. Psycho-Oncology, 12, 729–735.
Clarke, S. J., Yip, S., Brown, C., van Hazel, G. A., Ransom, D. T., Goldstein, D., et al. (2011). Single-agent irinotecan or FOLFIRI as second-line chemotherapy for advanced colorectal cancer; results of a randomised phase II study (DaVINCI) and meta-analysis. European Journal of Cancer, 47(12), 1826–1836.
Kaasa, S., Brenne, E., Lund, J. A., Fayers, P., Falkmer, U., Holmberg, M., et al. (2006). Prospective randomised multicenter trial on single fraction radiotherapy (8Gy x1) versus multiple fractions (3Gy x10) in the treatment of painful bone metastases. Radiotherapy and Oncology, 29, 278–284.
Mystakidou, K., Tsilika, E., Parpa, E., Kalaidopoulou, O., Smyrniotis, V., & Vlahos, L. (2001). The EORTC core quality of life questionnaire (QLQ-C30, version 3.0) in terminally ill cancer patients under palliative care: Validity and reliability in a Hellenic sample. Internation Journal of Cancer, 94(1), 135–139.
Richardson, P. G., Barlogie, B., Berenson, J., Singhal, S., Jagannath, S., Irwin, D., et al. (2003). A phase 2 study of Bortezomib in relapsed, refractory myeloma. New England Journal of Medicine, 348, 2609–2617.
Singer, S., Wollbrück, D., Wulke, C., Dietz, A., Klemm, E., Oeken, J., et al. (2009). Validation of the EORTC QLQ-C30 and EORTC QLQ-H&N35 in patients with laryngeal cancer after surgery. Head and Neck, 31(1), 64–76.
Smith, A. B., King, M. T., Butow, P., Luckett, T., Grimison, P., Toner, G. C., et al. (2013). The prevalence and correlates of supportive care needs in testicular cancer survivors: A cross-sectional study. Psycho-Oncology, 22(11), 2557–2564.
Tebbutt, N. C., Cummins, M. M., Sourjina, T., Strickland, A. H., Van Hazel, G., Ganju, V., et al. (2010). Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: The AGITG ATTAX trial. British Journal of Cancer, 102(3), 475–481.
Tebbutt, N. C., Parry, M. M., Zannino, D., Strickland, A. H., Van Hazel, G. A., Pavlakis, N., et al. (2013). Docetaxel plus cetuximab as second-line treatment for docetaxel-refractory oesophagogastric cancer: The AGITG ATTAX2 trial. British Journal of Cancer, 108(4), 771–774.
Teckle, P., Peacock, S., McTaggart-Cowan, H., van der Hoek, K., Chia, S., Melosky, B., & Gelmon, K. (2011). The ability of cancer-specific and generic preference-based instruments to discriminate across clinical and self-reported measures of cancer severities. Health and Quality of Life Outcomes, 9, 106.
Velikova, G., Booth, L., Smith, A. B., Brown, P. M., Lynch, P., Brown, J., & Selby, P. J. (2004). Measuring quality of life in routine oncology practice improves communication and patient well-being: A randomized controlled trial. Journal of Clinical Oncology, 22(4), 714–724.
Velikova, G., Sheppard, S., Campbell, L., Smith, A., Awad, N., Selby, P., et al. (2008). Randomized trial of quality-of-life measurement in oncology practice: Do oncologists need to know? ASCO annual meeting (Vol. 26, pp. 9586). Chicago: Journal of Clinical Oncology.
Whistance, R. N., Conroy, T., Chie, W., Costantini, A., Sezer, O., Koller, M., et al. (2009). Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. European Journal of Cancer, 45(17), 3017–3026.
Klepstad, P., Dale, O., Kaasa, S., Zahlsen, K., Aamo, T., Fayers, P., & Borchgrevink, P. C. (2003). Influences on serum concentrations of morphine, M6G and M3G during routine clinical drug monitoring: A prospective survey in 300 adult cancer patients. Acta Anaesthesiologica Scandinavica, 47, 725–731.
Sintonen, H. (2001). The 15D instrument of health-related quality of life: Properties and applications. Annals of Medicine, 33, 328–336.
Rowen, D., Brazier, J. E., Young, T., Gaugris, S., Craig, B. M., King, M. T., & Velikova, G. (2011). Deriving a preference-based measure for cancer using the EORTC-QLQC30. Value in Health, 14(5), 721–731.
Costa, D. S., Aaronson, N. K., Fayers, P. M., Grimison, P., Janda, M., Pallant, J. F., et al. (2014). Deriving a preference-based utility measure for cancer patients from the EORTC QLQ-C30: A confirmatory versus exploratory approach. Patient Related Outcome Measures, 5, 119–129.
Costa, D. S. J., King, M. T., Aaronson, N. K., Brazier, J. E., Cella, D., Grimison, P., et al. (2014). Do cancer patients distinguish between the importance and severity of their quality of life concerns? Annual Conference for the international society for quality of life research (Vol. 23, pp. 122). Berlin, Germany: Quality of Life Research.
McTaggart-Cowan, H., Teckle, P., & Peacock, S. (2013). Mapping utilities from cancer-specific health-related quality of life instruments: A review of the literature. Expert Review of Pharmacoeconomics & Outcomes Research, 13(6), 753–765.
Fayers, P. M., & Hays, R. D. (2014). Should linking replace regression when mapping from profile-based measures to preference-based measures? Value in Health, 17, 261–265.
Brazier, J. E., Yang, Y., Tsuchiya, A., & Rowen, D. L. (2010). A review of studies mapping (or cross walking) non preference based measures of health to generic preference-based measures. The European Journal of Health Economics, 11, 215–225.
Scott, N. W., Fayers, P., Aaronson, N., Bottomley, A., de Graeff, A., Groenvold, M., et al. (2008). EORTC QLQ-C30 Reference Values. Brussels: European Organisation for Research and Treatment of Cancer (EORTC).
Jim, H. S. L., Phillips, K. M., Chait, S., Faul, L. A., Popa, M. A., Lee, Y. H., et al. (2012). Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. Journal of Clinical Oncology, 30(29), 3578–3587.
Park, J. H., & Bae, S. H. (2012). A meta-analysis of chemotherapy related cognitive impairment in patients with breast cancer. Journal of Korean Academy of Nursing, 42(5), 644–658.
Acknowledgments
The MAUCa Consortium, in addition to those named as authors, consists of the following members, all of whom made some contribution to the research reported in this paper: Stuart Peacock, Helen McTaggart-Cowan, Julie Pallant and Deborah Street. We would also like to thank the following people for their generosity in contributing data for secondary analysis: U. Abacioğlu, J. Arraras, J. Blazeby, W.-C. Chie, S. Clarke, S. Kaasa, P. Klepstad, Millennium Pharmaceuticals, K. Mystakidou, S. Peacock, R. Schwarz, N. Scott, N. Tebbutt, G. Velikova and the Australian Gastro-Intestinal Trials Group. This research was supported by a National Health and Medical Research Council (Australia) Project Grant (632662). A/Professor Janda was supported by a NHMRC career development award 1045247. Professor King was supported by the Australian Government through Cancer Australia.
Funding
This research was supported by a National Health and Medical Research Council (Australia) Project Grant (632662). Professor King was supported by the Australian Government through Cancer Australia. Dr. Norman was supported by a NHMRC early career research fellowship (1069732).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they do not have conflicts of interest.
Ethical approval
The study was approved by the University of Sydney Human Research Ethics Committee, approval number 2012/2444. All study procedures involving human participants were in accordance with the ethical standards of institutional and national research committees and with the 1964 Helsinki Declaration and its later amendments and comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
On behalf of the MAUCa Consortium.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
King, M.T., Costa, D.S.J., Aaronson, N.K. et al. QLU-C10D: a health state classification system for a multi-attribute utility measure based on the EORTC QLQ-C30. Qual Life Res 25, 625–636 (2016). https://doi.org/10.1007/s11136-015-1217-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-1217-y