Abstract
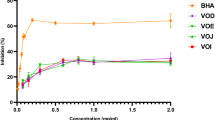
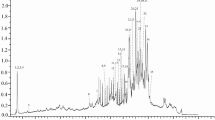
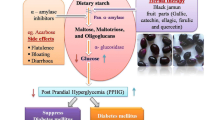
Red arils of Pithecellobium dulce fruit, commonly known as guamuchil, show high antioxidant (AOx) and α-glucosidase inhibitory (IαG) activities, which have been mainly associated with the content of unknown anthocyanins. In this study, the AOx (i.e., DPPH and ABTS as Trolox equivalents, μmol TE/g) and IαG (as half-maximal inhibitory concentration, IC50, mg/mL) activities of the anthocyanin-rich fraction (ARF) obtained from red arils were contrasted with those of the methanol extract (ME), and the main ARF anthocyanins were characterized by HPLC-DAD-ESI-MS, GC-MS and 1H-NMR. The AOx and IαG values of the ARF (DPPH = 597.8; ABTS = 884.01; IαG = 0.06) were better than those of the ME (DPPH = 41.5; ABTS = 142.3; IαG = 17.5); remarkably, the ARF IαG value was about 42 times lower than that of acarbose. The main anthocyanins in ARF were pelargonidin 3-O-glucoside and cyanidin 3-O-glucoside. Thus, the consumption of red P. dulce arils could provide health benefits for prevention/treatment of chronic degenerative diseases such as diabetes.

Similar content being viewed by others
Abbreviations
- AOx:
-
Antioxidant activity
- IαG:
-
α-glucosidase inhibitory
- ME:
-
Methanol extract
- aME:
-
Acidified methanol extract
- ARF:
-
Anthocyanin-rich fraction
- C3G:
-
Cyanidin 3-O-glucoside
- P3G:
-
Pelargonidin 3-O-glucoside
References
Parrotta J (1991) Pithecellobium dulce (Roxb.) Benth. Guamúchil, Madras thorn., vol SO-ITS-SM-40. Forest Service, North Carolina
UNAM (2009) Biblioteca Digital de la Medicina Tradicional Mexicana. Universidad Nacional Autónoma de México. http://www.medicinatradicionalmexicana.unam.mx/termino.php?l=1&t=diarrea&letra=D&opcion=D&id=2968. Accessed 23 Feb 2018
Ponmozhi P, Geethá M, Saravana Kumar M et al (2011) Extraction of anthocyanin and analysing its antioxidant properties from Pithecellobium dulce fruit pericarp. Asian J Pharm Clin Res 4(Suppl. 1):41–45 https://innovareacademics.in/journal/ajpcr/Vol44Suppl41/377.pdf
Pío-León JF, Díaz-Camacho S, Montes-Avila J, López-Angulo G, Delgado-Vargas F (2013) Nutritional and nutraceutical characteristics of white and red Pithecellobium dulce (Roxb.) Benth fruits. Fruits 68(5):397–408. https://doi.org/10.1051/fruits/2013084
Wall-Medrano A, Gonzalez-Aguilar GA, Loarca-Pina GF et al (2016) Ripening of Pithecellobium dulce (Roxb.) Benth. [guamuchil] fruit: physicochemical, chemical and antioxidant changes. Plant Foods Hum Nutr 71(4):396–401
Andersen ØM, Jordheim M (2006) The anthocyanins. In: Andersen ØM, Markham KR (eds) Flavonoids. Chemistry, Biochemistry and Applications, 1st edn. Taylor and Francis Group, New York, pp 471–551. https://doi.org/10.1201/9781420039443.ch10
Rodriguez-Saona LE, Wrolstad RE (2001) Extraction, isolation, and purification of anthocyanins. Curr Protoc Food Analyt Chem F1.1.1–F1.1.11. https://doi.org/10.1002/0471142913.faf0101s00
Hema A, Palé E, Duez P et al (2012) Two diglucosylated anthocyanins from Combretum paniculatum flowers. Nat Sci 4(3):166–169. https://doi.org/10.4236/ns.2012.43024
Tatsuzawa F, Saito N, Yukawa T, Honda T, Shinoda K, Kato K, Miyoshi K (2014) Acylated cyanidin 3,7-diglucosides in the red-purple flowers of Sophronitis wittigiana (Orchidaceae). J Jpn Soc Hortic Sci 83(1):64–71. https://doi.org/10.2503/jjshs2501.CH-2084
Qin CG, Li Y, Niu WN, Ding Y, Shang XY, Xu CL (2011) Composition analysis and structural identification of anthocyanins in fruit of waxberry. Czech J Food Sci 29(2):171–180. https://doi.org/10.17221/17177/12010-CJFS
López-Angulo G, Montes-Avila J, Díaz-Camacho SP, Vega-Aviña R, Báez-Flores ME, Delgado-Vargas F (2016) Bioactive components and antimutagenic and antioxidant activities of two Echeveria DC. species. Ind Crop Prod 85:38–48. https://doi.org/10.1016/j.indcrop.2016.1002.1044
Durst RW, Wrolstad RE (2001) Separation and characterization of anthocyanins by HPLC. Curr Protoc Food Analyt Chem F1.3.1–F1.3.13. https://doi.org/10.1002/0471142913.faf0103s00
da Silva Pinto M, Kwon Y-I, Apostolidis E, Lajolo FM, Genovese MÍ, Shetty K (2008) Functionality of bioactive compounds in Brazilian strawberry (Fragaria x ananassa Duch.) cultivars: evaluation of hyperglycemia and hypertension potential using in vitro models. J Agric Food Chem 56(12):4386–4392
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30. https://doi.org/10.1016/S0023-6438(1095)80008-80005
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237
Aguilar O, Hernandez-Brenes C (2015) Use of modified phenolic thyme extracts (Thymus vulgaris) with reduced polyphenol oxidase substrates as anthocyanin color and stability enhancing agents. Molecules 20(12):22422–22434
Jansom C, Bhamarapravati S, Itharat A (2008) Major anthocyanin from ripe berries of Cleistocalyx nervosum var. paniala. Thammasat Med J 8(3):394–370 https://www.researchgate.net/publication/242190711
Cui C, Zhang S, You L, Ren J, Luo W, Chen W, Zhao M (2013) Antioxidant capacity of anthocyanins from Rhodomyrtus tomentosa (Ait.) and identification of the major anthocyanins. Food Chem 139(1–4):1–8
Kajdžanoska M, Gjamovski V, Stefova M (2010) HPLC-DAD-ESI-MSn identification of phenolic compounds in cultivated strawberries from Macedonia. Maced J Chem Chem Eng 29(2):181–194 http://www.mjcce.org.mk/index.php/MJCCE/article/view/165
Berardini N, Schieber A, Klaiber I, Beifuss U, Carle R, Conrad J (2005) 7-O-Methylcyanidin 3-O-β-D-galactopyranoside, a novel anthocyanin from mango (Mangifera indica L.) cv. ‘Tommy Atkins’ peels. Z Naturforsch B 60:801–804. https://doi.org/10.1515/znb-2005-0718
Lee JH, Kang NS, Shin S-O, Shin SH, Lim SG, Suh DY, Baek IY, Park KY, Ha TJ (2009) Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem 112(1):226–231. https://doi.org/10.1016/j.foodchem.2008.1005.1056
Pérez MJ, Cuello AS, Zampini IC, Ordoñez RM, Alberto MR, Quispe C, Schmeda-Hirschmann G, Isla MI (2014) Polyphenolic compounds and anthocyanin content of Prosopis nigra and Prosopis alba pods flour and their antioxidant and anti-inflammatory capacities. Food Res Int 64:762–771
Rasouli H, Hosseini-Ghazvini SM, Adibi H et al (2017) Differential alpha-amylase/alpha-glucosidase inhibitory activities of plant-derived phenolic compounds: a virtual screening perspective for the treatment of obesity and diabetes. Food Funct 8(5):1942–1954
Bae IY, An JS, Oh IK, Lee HG (2017) Optimized preparation of anthocyanin-rich extract from black rice and its effects on in vitro digestibility. Food Sci Biotechnol 26(5):1415–1422. https://doi.org/10.1007/s10068-10017-10188-x
Adisakwattana S, Charoenlertkul P, Yibchok-Anun S (2009) Alpha-glucosidase inhibitory activity of cyanidin-3-galactoside and synergistic effect with acarbose. J Enzyme Inhib Med Chem 24(1):65–69
Akkarachiyasit S, Charoenlertkul P, Yibchok-Anun S et al (2010) Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal alpha-glucosidase and pancreatic alpha-amylase. Int J Mol Sci 11(9):3387–3396
He H, Lu YH (2013) Comparison of inhibitory activities and mechanisms of five mulberry plant bioactive components against alpha-glucosidase. J Agric Food Chem 61(34):8110–8119
Matsui T, Ueda T, Oki T, Sugita K, Terahara N, Matsumoto K (2001) Alpha-glucosidase inhibitory action of natural acylated anthocyanins. 1. Survey of natural pigments with potent inhibitory activity. J Agric Food Chem 49(4):1948–1951
Martin Bueno J, Ramos-Escudero F, Sáez-Plaza P et al (2012) Analysis and antioxidant capacity of anthocyanin pigments. Part I: general considerations concerning polyphenols and flavonoids. Crit Rev Anal Chem 42(2):102–125. https://doi.org/10.1080/10408347.10402011.10632312
Jakobek L, Šeruga M, Medvidović-Kosanović M et al (2007) Anthocyanin content and antioxidant activity of various red fruit juices. Deut Lebensm-Rundsch 103:58–64 https://bib.irb.hr/datoteka/210741.Jakobek_et_al_DLR_10322007.10322058-10322064.PDF
Lee SG, Vance TM, Nam TG, Kim DO, Koo SI, Chun OK (2015) Contribution of anthocyanin composition to total antioxidant capacity of berries. Plant Foods Hum Nutr 70(4):427–432
Delazar A, Khodaie L, Afshar J, Nahar L, Sarker S (2010) Isolation and free-radical-scavenging properties of cyanidin 3-O-glycosides from the fruits of Ribes biebersteinii Berl. Acta Pharma 60(1):1–11
Kähkönen MP, Heinonen M (2003) Antioxidant activity of anthocyanins and their aglycons. J Agric Food Chem 51(3):628–633
Kay CD, Pereira-Caro G, Ludwig IA, Clifford MN, Crozier A (2017) Anthocyanins and flavanones are more bioavailable than previously perceived: a review of recent evidence. Annu Rev Food Sci Technol 8:155–180
Gowd V, Bao T, Wang L, Huang Y, Chen S, Zheng X, Cui S, Chen W (2018) Antioxidant and antidiabetic activity of blackberry after gastrointestinal digestion and human gut microbiota fermentation. Food Chem 269:618–627
Acknowledgements
Authors acknowledge this research was partially funded by CONACYT-Mexico and PROFAPI-Universidad Autonoma de Sinaloa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare they have no conflict of interest.
Rights and permissions
About this article
Cite this article
López-Angulo, G., Montes-Avila, J., Sánchez-Ximello, L. et al. Anthocyanins of Pithecellobium dulce (Roxb.) Benth. Fruit Associated with High Antioxidant and α-Glucosidase Inhibitory Activities. Plant Foods Hum Nutr 73, 308–313 (2018). https://doi.org/10.1007/s11130-018-0693-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-018-0693-y