Abstract
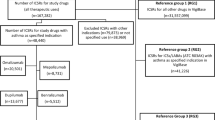
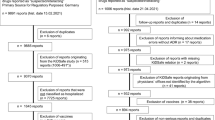
Background Information about safety issues from use of asthma medications in children is limited. Spontaneous adverse drug reaction (ADR) reports can provide information about serious and rarely occurring ADRs in children. Objective To characterize paediatric ADRs reported for asthma medications licensed for paediatric use. Setting Spontaneous ADR reports located in the European ADR database, EudraVigilance. Method ADRs reported for asthma medications licensed for paediatric use from 2007 to 2011 were analysed. The included substances were beclometasone, budesonide, fenoterol, fluticasone, formoterol, mometasone, montelukast, salbutamol and terbutaline and the combinations of budesonide/formoterol, fenoterol/ipratropium and fluticasone/salmeterol. Main outcome measures Reported ADRs were categorized with respect to distribution on age, sex, type and seriousness of reported ADRs, medications and type of reporter. The unit of analysis was one ADR. Results We located 326 spontaneous reports corresponding to 774 ADRs for the included asthma medications. Approximately 85 % of reported ADRs were serious including six fatal cases. In total, 57 % of ADRs were reported for boys. One quarter of all ADRs occurred in children up to 1 year of age. Physicians reported the majority of ADRs. Across medicines, the majority of reported ADRs were of the type “psychiatric disorders” (13 % of total ADRs), followed by “respiratory, thoracic and mediastinal disorders” (10 % of total ADRs) and “skin and subcutaneous disorders” (9 % of total ADRs). The largest number of ADRs was reported for budesonide (21 % of total ADRs), followed by salbutamol (20 % of total ADRs) and fluticasone (19 % of total ADRs). For salbutamol, the largest numbers of serious ADRs were “tachycardia”, “accidental exposure/incorrect dose administered” and “respiratory failure”. Conclusion Only a few ADRs from use of asthma medications in children were identified in the EudraVigilance ADR database, but a large majority of these were serious including fatal cases.
Similar content being viewed by others
References
Grover C, Armour C, Van Aspersen PP, Moles R, Saini B. Medication use in children with asthma: not a child size problem. J Asthma. 2011;48:1085–103.
Thrane N, Sørensen HT. A one-year population-based study of drug prescriptions for Danish children. Acta Paediatr. 1999;88:1131–6.
Aagaard L, Hansen EH. Adverse drug reactions associated with asthma medications in children: systematic review of clinical trials. Int J Clin Pharm. 2014;36:243–52.
De Vries TW, de Langen-Wouterse JJ, van Puijenbroek E, Duiverman EJ, de Jong-Van den Berg LTW. Reported adverse drug reactions during the use of inhaled steroids in children with asthma in the Netherlands. Eur J Clin Pharmacol. 2006;62:343–6.
de Vries TW, de Langen-Wouterse JJ, de Jong-Van den Berg LTW, Duiverman EJ. Hypertrichosis as a side effect of inhaled steroids in children. Pediatr Pulmonol. 2007;42:370–3.
Aagaard L, Hansen EH. Information about ADRs explored by pharmacovigilance approaches: a qualitative review of studies on antibiotics, SSRIs and NSAIDs. BMC Clin Pharmacol. 2009;9:4. doi:10.1186/1472-6904-9-4.
Aagaard L, Christensen A, Hansen EH. Information about adverse drug reactions reported in children: a qualitative review of studies. Br J Clin Pharmacol. 2010;70:481–91.
Aagaard L, Hansen EH. Adverse drug reactions in children reported by European consumers from 2007 to 2011. Int J Clin Pharm. 2014;36:295–302.
EU directive of the European Parliament and of the Council amending Directive 2001/83/EC as regards information to the general public on medicinal products subject to medical prescription. COM (2012) 48 final. Brussels, 10.2.2012. http://eur-lex.europa.eu/search.html?DTN=0083&or0=DTS%3D3%2CDTS%3D0&qid=1408017970211&type=advanced&DTS_SUBDOM=ALL_ALL&or1=DTT%3DL&SUBDOM_INIT=ALL_ALL&DTS_DOM=ALL&instInvStatus=ALL&DTC=false&DTA=2001&sortOne=TI_SORT&sortOneOrder=desc. Accessed 14 Aug 2014.
Hansen EH, Launsø L. Clinical trials: towards a different model. J Pharm Clin. 1987;6:67–74.
European Medicines Agency. EudraVigilance access policy for medicines for human use. EMA/759287/2009 corr. Published 23 August 2011. http://www.ema.europa.eu/ema/doc_index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500170699&murl=menus/document_library/document_library.jsp&mid=0b01ac058009a3dc. Accessed 14 Aug 2014.
Official Journal of the European communities. Regulation (EC) No. 1049/2001 of the European Parliament and of the Council of 30 May 2001 regarding public access to European Parliament, Council and Commission documents. L 145/43. http://eur-lex.europa.eu/legal-content/DA/ALL/;ELX_SESSIONID=V1qWTskGzb3Hbn0RHNT2yYTtjGnrfzXDTpjbyP1TTrfvK5hG8MkM!-1016850525?uri=CELEX:32001R1049. Accessed 14 Aug 2014.
MedDRA. http://www.meddra.org/. Accessed 14 Aug 2014.
Volume 9. Pharmacovigilance: medicinal products for human use and veterinary products. http://ec.europa.eu/enterprise/pharmaceuticals/eudralex/homev9.htm. Accessed 14 Aug 2014.
National Heart Lung and Blood Institute. Guidelines for the diagnosis and management of asthma (EPR-3). http://www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed 14 Aug 2014.
Cereza G, Garcia Doladé N, Laporte JR. Nightmares induced by montelukast in children and adults. Eur Respir J. 2012;40:1574–5.
Arellano FM, Arana A, Wentworth CE, Vidaurre CF, Chipps BE. Prescription patterns for asthma medications in children and adolescents with health care insurance in the United States. Pediatr Allergy Immunol. 2011;22:469–76.
Pagotto C, Varallo F, Mastroianni P. Strategies to improve adverse drug reaction reporting: a critical and systematic review. Int J Technol Assess Health Care. 2013;29:410–7.
Acknowledgments
We would like to thank the European Medicines Agency for providing data.
Funding
No sources of funding were used to assist in the preparation of this study.
Conflicts of interest
The authors report no conflict of interest with respect to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lise Aagaard and Ebba Holme have contributed significantly to the manuscript.
Rights and permissions
About this article
Cite this article
Aagaard, L., Hansen, E.H. Paediatric adverse drug reactions following use of asthma medications in Europe from 2007 to 2011. Int J Clin Pharm 36, 1222–1229 (2014). https://doi.org/10.1007/s11096-014-0020-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-014-0020-0