Abstract
Purpose
To understand the mechanisms of secondary drying of spray-dried dispersion (SDD) drug product and establish a model to describe the fate of organic solvents in such a product.
Methods
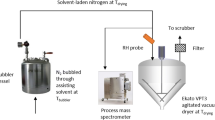
The experimental approach includes characterization of the SDD particles, drying studies of SDD using an integrated weighing balance and mass spectrometer, and the subsequent generation of the drying curve. The theoretical approach includes the establishment of a Fickian diffusion model.
Results
The kinetics of solvent removal during secondary drying from the lab scale to a bench scale follows Fickian diffusion model. Excellent agreement is obtained between the experimental data and the prediction from the modeling.
Conclusions
The diffusion process is dependent upon temperature. The key to a successful scale up of the secondary drying is to control the drying temperature. The fate of primary solvents including methanol and acetone, and their potential impurity such as benzene can be described by the Fickian diffusion model. A mathematical relationship based upon the ratio of diffusion coefficient was established to predict the benzene concentration from the fate of the primary solvent during the secondary drying process.















Similar content being viewed by others
Abbreviations
- API:
-
Active pharmaceutical ingredient
- D:
-
Diffusion coefficient
- DSC:
-
Differential scanning calorimetry
- GC:
-
Gas chromatography
- HPMCAS:
-
Hydroxypropylmethylcellulose (hypromellose) acetate succinate
- ICH:
-
International conference of harmonisation
- MS:
-
Mass spectroscopy
- PSD:
-
Particle size distribution
- PVP K30:
-
Polyvinylpyrrolidone K30
- PXRD:
-
Powder x-ray diffraction
- SDD:
-
Spray dried dispersion
- SEM:
-
Scanning electron microscope
- Tg :
-
Glass transition temperature
- αAB :
-
Relative diffusion rate of solvent A with respect to solvent B
- β:
-
Diffusion parameter
References
Paudel A, Worku Z, Meeus J, Guns S, Mooter GV. Manufacturing of solid dispersions of poorly water soluble drugs by spray drying: formulation and process considerations. Int J Pharm. 2013;453:263–84.
Mooter GVD. The use of amorphous solid dispersions: a formulation strategy to overcome poor solubility and dissolution rate. Drug Discov Today: Technol. 2012;9(2):79–85.
Ray R. Addressing solubility challenges: using effective technology & problem-solving for delivery solutions. Drug Dev Deliv. 2012;12(6):26–8.
Dobry DE, Settell DM, Baumann JM, Ray RJ, Graham LI, Beyerinck RA. A model-based methodology for spray-drying process development. J Pharm Innov. 2009;4:133–42.
McCabe WL, Smith JC, Harriott P. Unit Operations of Chemical Engineering. Fifth Edition, McGraw-Hill; 1993; 803.
ICH, Impurities: guideline for residue solvents Q3C(R5). ICH; February 2011.
Yue H, Nicholson S, Young J, Hsieh D, Ketner R, Hall R. Sackett J, Bank E, Castoro J, Randazzo M. Development of a control strategy for benzene impurity in HPMCAS-stabilized spray-dried dispersion drug products using a science-based and risk-based approach. Unpublished.
Matteucci S, Yampolskii Y, Freeman BD, Pinnau I. Transport of gases and vapors in glassy and rubbery polymers. In: Yampolskii Y, Pinnau I, Freeman BD. Editors. Materials science of membranes for gas and vapor separation. John Wiley & Sons, Ltd, 2006
Gamble JF, Ferreira AP, DiMemmo L, Martin K, Mathias N, Schild R, et al. Application of imaging based tools for the characterisation of hollow spray dried amorphous dispersion particles. Int J Pharm. 2014;465:210–7.
Vickery RD, Stefanski KJ, Su C-C, Hageman MJ, Vig BS, Betigeri S.Bioavailable compositions of amorphous piperidinyl compounds. Geneva (Switzerland): World Intellectual Property Organization; 2013 Jan. International Publication Number: WO 2013/013114 A1.
Crank J. The Mathematics of Diffusion. 2nd ed. London (England): Claredon Press; 1975.
Vrentas JS, Vrentas CM. Diffusion and Mass Transfer. Florida: Taylor & Francis/CRC; 2013.
Basmadjian D. The Art of Modeling in Science and Engineering. Chapman & Hall/CRC: Florida; 1999.
Sperling LH. Physical polymer science. 3rd ed. New York (USA): Wiley Interscience; 2001.
Dounce SM, Mundy J, Dai HL. Crystallization at the glass transition in super-cooled thin film of methanol, J Chem Physics. 200; 126, 191111.
Duda JL. Molecular diffusion in polymeric systems. Pure Appl Chem. 1985;57(11):1681–90.
Cussler EL. Diffusion, mass transfer in fluid system. 2nd ed. Cambride (UK): Cambrideg University Press; 1997.
Flanigan EM et al. Silicalite, a new hydrophobic crystalline silica molecular sieve. Nature. 1978;271:512–6.
Cosseron AF et al. Adsorption of solvent organic compounds in pure silica CHA, *BEA, MFI and STT-type zeolite. Microporous Mesoporous Mater. 2013;173:173147–54.
Chandra P, Koros WJ. Sorption and tranport of methanol in poly(ethylene terephthalate). Polymer. 2009;50:236–44.
Acknowledgments
The authors are grateful for the Senior Leadership Team and the project team members at Bristol-Myers Squibb Co. for providing support to accomplish this work. The project team members include Kyle Martin, Dr. Neil Mathias, Dr. Balvinder Vig, Lynn DiMemmo, Dr. Steve Wang and Dr. Steven Chan. The technical team members at Bend Research are greatly acknowledged for their generous technical support for secondary drying. Special thanks are given to Mike Ashton at Intertek Pharmaceutical Services (Manchester, UK) for conducting cryogenic scanning electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hsieh, D.S., Yue, H., Nicholson, S.J. et al. The Secondary Drying and the Fate of Organic Solvents for Spray Dried Dispersion Drug Product. Pharm Res 32, 1804–1816 (2015). https://doi.org/10.1007/s11095-014-1577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1577-y