Abstract
Purpose
To evaluate the plant phenolics, malabaricone B (mal B) and malabaricone C (mal C) in healing stomach ulcer by modulating angiogenesis.
Materials and Methods
Male Swiss albino mice, ulcerated with indomethacin (18 mg/kg, p. o., single dose) were treated up to 7 days with different doses of mal B or mal C. The healing capacities of the drugs and their effects on the angiogenic parameters were assessed.
Results
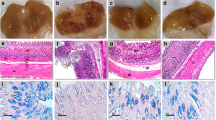
Maximum ulceration, observed on the 3rd day after indomethacin administration was effectively healed by mal B and mal C (each 10 mg/kg, p. o. × 3 days), the latter showing equivalent potency (~78% p < 0.001) as that of Omez (3 mg/kg, p. o. × 3 days) and misoprostol (10 μg/kg, p. o. × 3 days). Compared to the untreated mice, those treated with mal B or mal C respectively for 3 days increased the mucosal EGF level (139 and 178%, p < 0.001), the serum VEGF level (56%, p < 0.01 and 95%, p < 0.001) and microvessels formation (37%, p < 0.05 and 62%, p < 0.01), while reducing the serum endostatin level (37%, p < 0.05 and 61%, p < 0.01). The relative healing capacities of mal B and mal C correlated well with their respective abilities to modulate the angiogenic factors. The healing by Omez and misoprostol was not due to improved angiogenesis.
Conclusions
The drugs, mal B and mal C could effectively heal indomethacin-induced stomach ulceration in mice by promoting angiogenesis.





Similar content being viewed by others
References
C. Hawkins, and G. W. Hanks. The gastroduodenal toxicity of nonsteroidal anti-inflammatory drugs. A review of the literature. J. Pain Symp. Manag 20:140–151 (2000).
M. J. Lancaster-Smith, M. R. Jaderberg, and D. A. Jackson. Ranitidine in the treatment of non-steroidal anti-inflammatory drug associated gastric and duodenal ulcers. Gut 32:252–256 (1991).
F. Halter, A. Schamassman, B. M. Peskar, and A. S. Tarnawski. Cyclooxygenase-2 implications on maintenance of gastric mucosal integrity and ulcer healing: controversies and perspectives. Gut 49:443–453 (2001).
H. Hirose, K. Takeuchi, and S. Okabe. Effect of indomethacin on gastric mucosal blood flow around acetic acid-induced gastric ulcers in rats. Gastroenterol 100:1259–1265 (1991).
A. Tarnawski, T. G. Douglass, J. Stachura, and W. J. Krause. Quality of gastric ulcer healing: histological ultrastructural assessment. Aliment Pharmacol. Therap 5(Suppl 1):79–90 (1991).
M. H. McGrath, and J. M. I. Emery. The effect of inhibition of angiogenesis in granulation tissue on wound healing and the fibroblast. Ann. Plast Surg 15:106–119 (1985).
B. S. Patro, A. K. Bauri, S. Mishra, and S. Chattopadhyay. Antioxidant activity of Myristica malabarica extracts and its constituents. J. Agric. Food Chem 53:6912–6918 (2005).
K. Biswas, U. Bandyopadhyay, I. Chattopadhyay, A. Varadaraj, E. Ali, and R. K. Banerjee. A novel antioxidant and antiapoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J. Biol. Chem 278:10993–11001 (2003).
D. Dokmeci, M. Akpolat, N. Aydogu, L. Doganay, and F. N. Turan. L-Carnitine inhibits ethanol-induced gastric mucosal injury in rats. Pharmacol. Rep 57:481–488 (2005).
D. Banerjee, B. Maity, S. K. Bandyopadhyay, A. K. Bauri, and S. Chattopadhyay. Gastroprotective properties of Myristica malabarica against indomethacin-induced stomach ulceration: a mechanistic exploration. J. Pharm. Phamacol. 59:1555–1565 (2007).
K. Yokoi, P. H. Thaker, S. Y. Robert, R. Rebhun, D. H. Nam, J. He, S. J. Kim, J. L. Abbruzzese, S. R. Hamilton, and I. J. Fidler. Dual inhibition of epidermal growth factor receptor and vascular endothelial growth factor receptor phosphorylation by AEE 788 reduces growth and metastasis of human colon carcinoma in an orthotopic nude mouse model. Cancer Res 65:3716–3725 (2005).
N. Weidner, J. P. Semple, W. R. Welch, and J. Folkman. Tumor angiogenesis and metastasis: correlation in invasive breast carcinoma. New Engl. J. Med 324:1–8 (1991).
A. Schmassmann. Mechanisms of ulcer healing and effects of nonsteroidal anti-inflammatory drugs. Am. J. Med 104:43S–51S (1998).
M. K. Jones, H. Wang, B. M. Peskar, E. Levin, R. M. Itani, I. J. Sarfeh, and A. S. Tarnawski. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat. Med 5:1418–1423 (1999).
J. L. Wallace, and D. N. Granger. The cellular and molecular basis of gastric mucosal defense. FASEB J 10:731–740 (1996).
R. Lad, and D. Armstrong. Management of nonsteroidal anti-inflammatory drug-induced gastroduodenal disease by acid suppression. Can. J. Gastroenterol 13:135–142 (1999).
S. G. Chiverton, D. W. Burget, B. J. Salena, and R. H. Hunt. Does misoprostol given as a single large dose improve its antisecretory effect? Aliment Pharmacol. Ther 3:403–407 (1989).
W. M. Hui, B. W. Chen, C. H. Cho, C. T. Luk, and S. K. Lam. Role of gastric mucosal blood flow in cytoprotection. Digestion 48:113–120 (1991).
R. J. Coffey, M. Romano, and J. Goldenring. Roles for transforming growth factor-alpha in the stomach. J. Clin. Gastroenterol 2(Suppl 1):S36–S39 (1995).
L. R. Johnson, and S. A. McCormack. Regulation of gastrointestinal mucosal growth. In L. R. Johnson (ed.), Physiology of the Gastrointestinal Tract, (3rd edn), Raven, New York, 1994, pp. 611–641.
R. Pai, and A. S. Tarnawski. Signal transduction cascades triggered by EGF Receptor activation: relevance to gastric injury repair and ulcer healing. Dig. Dis. Sci 43(9 Suppl):14S–22S (1998).
A. Calabro, B. Orsini, A. Brocchi, M. Falchini, P. Fedi, and C. Surrenti. Gastric juice immunoreactive epidermal growth factor levels in patients with peptic ulcer disease. Am. J. Gastroenterol 85:404–407 (1990).
N. A. Wright, C. Pike, and G. Elia. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in the human gastrointestinal stem cells. Nature 343:82–85 (1990).
S. Szabo, and A. Vincze. Growth factors in ulcer healing: lessons from recent studies. J. Physiol. (London) 94:77–81 (2000).
J. Jin, and S. P. Kunapuli. Co-activation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. USA 95:8070–8074 (1998).
M. S. O’Reilly, T. Boehm, Y. Shing, N. Fukai, G. Vasios, W. S. Lane, E. Flynn, J. R. Birkhead, B. R. Olsen, and J. Folkman. Endostatin: An endogenous. inhibitor of angiogenesis and tumour growth. Cell 88:277–285 (1997).
N. Yamaguchi, B. Anand-Apte, M. Lee, T. Sasaki, N. Fukai, R. Shapiro, I. Que, C. Lowik, R. Timpl, and B. R. Olsen. Endostatin inhibits VEGF-induced endothelial cell migration and tumor growth independently of zinc binding. EMBO J 18:4414–4423 (1999).
E. Dejana, M. G. Lampugnani, M. Giorgi, et al. Von Willebrand factor promotes endothelial cell adhesion via an Arg-Gly-Asp-dependent mechanism. J. Cell Biol 109:367–375 (1989).
C. Wheeler-Jones, R. Abu-Ghazaleh, R. Cospedal, R. A. Houliston, J. Martin, and I. Zachary. Vascular endothelial growth factor stimulates prostacyclin production and activation of cytosolic phospholipase A2 icn endothelial cells via p42/p44 mitogen activated protein kinases. FEBS Left 420:28–32 (1997).
T. Tsuchida, Y. Tsukamoto, K. Segawa, H. Goto, and S. Hase. Effects of cimetidine and omeprazole on angiogenesis in granulation tissue of acetic acid-induced gastric ulcers in rats. Digestion 47:8–14 (1990).
W. A. Hoogerwerf, and P. J. Pasricha. Agents used for control of gastric acidity and treatment of peptic ulcers and gastroesophageal reflux disease. In J. G. Hardmann, L. E. Limbird, L. S. Goodman, and A. G. Gilman (eds.), Goodman & Gilman’s The Pharmacological Basis of Therapeutics, (10th ed), Mc Graw-Hill, New York, 2001, pp. 1005–1019.
A. Schmassmann, A. Tarnawski, B. M. Peskar, L. Varga, B. Flogerzi, and F. Halter. Influence of acid and angiogenesis on kinetics of gastric ulcer healing in rats: interaction with indomethacin. Am. J. Physiol. Gastrointest. Liver Physiol 268:G276–G285 (1995).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Banerjee, D., Maity, B., Bandivdeker, A.H. et al. Angiogenic and Cell Proliferating Action of the Natural Diarylnonanoids, Malabaricone B and Malabaricone C during Healing of Indomethacin-induced Gastric Ulceration. Pharm Res 25, 1601–1609 (2008). https://doi.org/10.1007/s11095-007-9512-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9512-0