Abstract
Introduction
Fluorescence guided surgery (FGS) with five-aminolevulinic acid (5-ALA) is expected to revolutionize neurosurgical care of patients with high-grade gliomas (HGG). After the recent landmark FDA approval, this optical agent is now available to neurosurgeons in the United States.
Methods
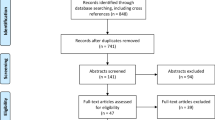
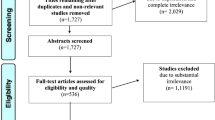
This review is designed to highlight the evidence for the use of 5-ALA in recurrent HGG surgery for the neurosurgical community. The manuscript was prepared in accordance with the PRISMA guidelines.
Results
Intra-operatively, a strong fluorescent signal is highly correlated with the presence of cellular tumor in recurrent HGG, giving it a high positive predictive value (PPV). Similar to what is observed in primary HGG surgery, false-negative results can occur if tumor cells do not emit fluorescence. In addition, false-positive fluorescence signals in tissues devoid of tumor cells can be observed more frequently in recurrent HGG compared to the primary setting. However, these areas overwhelmingly contain reactive/regressive tissue, resection of which is unlikely to cause functional deficits. The safety profile of 5-ALA is similarly favorable in primary and recurrent HGG.
Conclusions
5-ALA FGS is a powerful adjunct in the resection of recurrent HGG with a high PPV and favorable safety profile. It is therefore the authors’ opinion to routinely employ this fluorescent agent as a standard of care.
Similar content being viewed by others
References
Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS (2011) An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8. https://doi.org/10.3171/2011.2.JNS10998
Oppenlander ME, Wolf AB, Snyder LA, Bina R, Wilson JR, Coons SW, Ashby LS, Brachman D, Nakaji P, Porter RW, Smith KA, Spetzler RF, Sanai N (2014) An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg 120:846–853. https://doi.org/10.3171/2013.12.JNS13184
Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, Group AL-GS (2006) Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 7:392–401. https://doi.org/10.1016/S1470-2045(06)70665-9
Lau D, Hervey-Jumper SL, Chang S, Molinaro AM, McDermott MW, Phillips JJ, Berger MS (2016) A prospective Phase II clinical trial of 5-aminolevulinic acid to assess the correlation of intraoperative fluorescence intensity and degree of histologic cellularity during resection of high-grade gliomas. J Neurosurg 124:1300–1309. https://doi.org/10.3171/2015.5.JNS1577
Widhalm G, Minchev G, Woehrer A, Preusser M, Kiesel B, Furtner J, Mert A, Di Ieva A, Tomanek B, Prayer D, Marosi C, Hainfellner JA, Knosp E, Wolfsberger S (2012) Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg Rev 35:381–391. https://doi.org/10.1007/s10143-012-0374-5
Nabavi A, Thurm H, Zountsas B, Pietsch T, Lanfermann H, Pichlmeier U, Mehdorn M, Group ALARGS (2009) Five-aminolevulinic acid for fluorescence-guided resection of recurrent malignant gliomas: a phase ii study. Neurosurgery 65:1070–1076. https://doi.org/10.1227/01.NEU.0000360128.03597.C7 (discussion 1076–1077)
Utsuki S, Oka H, Sato S, Shimizu S, Suzuki S, Tanizaki Y, Kondo K, Miyajima Y, Fujii K (2007) Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol Med Chir 47:210–213 (discussion 213–214)
Kamp MA, Grosser P, Felsberg J, Slotty PJ, Steiger HJ, Reifenberger G, Sabel M (2012) 5-Aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir (Wien) 154:223–228. https://doi.org/10.1007/s00701-011-1200-5 (discussion 228)
Parvez K, Parvez A, Zadeh G (2014) The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int J Mol Sci 15:11832–11846. https://doi.org/10.3390/ijms150711832
Kumar AJ, Leeds NE, Fuller GN, Van Tassel P, Maor MH, Sawaya RE, Levin VA (2000) Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology 217:377–384. https://doi.org/10.1148/radiology.217.2.r00nv36377
Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ (2008) Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 9:453–461. https://doi.org/10.1016/S1470-2045(08)70125-6
Moher D, Liberati A, Tetzlaff J, Altman DG, Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151:264–269
Hoover JM, Nwojo M, Puffer R, Mandrekar J, Meyer FB, Parney IF (2013) Surgical outcomes in recurrent glioma clinical article. J Neurosurg 118:1224–1231. https://doi.org/10.3171/2013.2.Jns121731
Schucht P, Knittel S, Slotboom J, Seidel K, Murek M, Jilch A, Raabe A, Beck J (2014) 5-ALA complete resections go beyond MR contrast enhancement: shift corrected volumetric analysis of the extent of resection in surgery for glioblastoma. Acta Neurochir (Wien) 156:305–312. https://doi.org/10.1007/s00701-013-1906-7 (discussion 312)
Della Puppa A, Ciccarino P, Lombardi G, Rolma G, Cecchin D, Rossetto M (2014) 5-Aminolevulinic acid fluorescence in high grade glioma surgery: surgical outcome, intraoperative findings, and fluorescence patterns. Biomed Res Int 2014:232561. https://doi.org/10.1155/2014/232561
Idoate MA, Valle RD, Echeveste J, Tejada S (2011) Pathological characterization of the glioblastoma border as shown during surgery using 5-aminolevulinic acid-induced fluorescence. Neuropathology 31:575–582. https://doi.org/10.1111/j.1440-1789.2011.01202.x
Tonn JC, Stummer W (2008) Fluorescence-guided resection of malignant gliomas using 5-aminolevulinic acid: practical use, risks, and pitfalls. Clin Neurosurg 55:20–26
Stummer W, Tonn JC, Goetz C, Ullrich W, Stepp H, Bink A, Pietsch T, Pichlmeier U (2014) 5-Aminolevulinic acid-derived tumor fluorescence: the diagnostic accuracy of visible fluorescence qualities as corroborated by spectrometry and histology and postoperative imaging. Neurosurgery 74:310–319. https://doi.org/10.1227/Neu.0000000000000267
Hickmann AK, Nadji-Ohl M, Hopf NJ (2015) Feasibility of fluorescence-guided resection of recurrent gliomas using five-aminolevulinic acid: retrospective analysis of surgical and neurological outcome in 58 patients. J Neuro-oncol 122:151–160. https://doi.org/10.1007/s11060-014-1694-9
Kamp MA, Felsberg J, Sadat H, Kuzibaev J, Steiger HJ, Rapp M, Reifenberger G, Dibue M, Sabel M (2015) 5-ALA-induced fluorescence behavior of reactive tissue changes following glioblastoma treatment with radiation and chemotherapy. Acta Neurochir (Wien) 157:207–213. https://doi.org/10.1007/s00701-014-2313-4 (discussion 213–204)
Wachter D, Kallenberg K, Wrede A, Schulz-Schaeffer W, Behm T, Rohde V (2012) Fluorescence-guided operation in recurrent glioblastoma multiforme treated with bevacizumab-fluorescence of the noncontrast enhancing tumor tissue? J Neurol Surg A 73:401–406. https://doi.org/10.1055/s-0032-1304810
Tykocki T, Michalik R, Bonicki W, Nauman P (2012) Fluorescence-guided resection of primary and recurrent malignant gliomas with 5-aminolevulinic acid. Preliminary results. Neurol Neurochir Pol 46:47–51
Hefti M, von Campe G, Moschopulos M, Siegner A, Looser H, Landolt H (2008) 5-Aminolevulinic acid induced protoporphyrin IX fluorescence in high-grade glioma surgery: a one-year experience at a single institutuion. Swiss Med Wkly 138:180–185
Panciani PP, Fontanella M, Garbossa D, Agnoletti A, Ducati A, Lanotte M (2012) 5-Aminolevulinic acid and neuronavigation in high-grade glioma surgery: results of a combined approach. Neurocirugia (Astur) 23:23–28. https://doi.org/10.1016/j.neucir.2012.04.003
Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ (2000) Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: a prospective study in 52 consecutive patients. J Neurosurg 93:1003–1013. https://doi.org/10.3171/jns.2000.93.6.1003
Dietze A, Berg K (2005) ALA-induced porphyrin formation and fluorescence in synovitis tissue in-vitro and in vivo studies. Photodiagn Photodyn Ther 2:299–307. https://doi.org/10.1016/S1572-1000(05)00107-9
Dietel W, Bolsen K, Dickson E, Fritsch C, Pottier R, Wendenburg R (1996) Formation of water-soluble porphyrins and protoporphyrin IX in 5-aminolevulinic-acid-incubated carcinoma cells. J Photochem Photobiol B 33:225–231. https://doi.org/10.1016/1011-1344(95)07249-7
Polo CF, Frisardi AL, Resnik ER, Schoua AEM, Batlle AMD (1988) Factors influencing fluorescence-spectra of free porphyrins. Clin Chem 34:757–760
Valle RD, Solis ST, Gastearena MAI, de Eulate RG, Echavarri PD, Mendiroz JA (2011) Surgery guided by 5-aminolevulinic fluorescence in glioblastoma: volumetric analysis of extent of resection in single-center experience. J Neuro-Oncol 102:105–113. https://doi.org/10.1007/s11060-010-0296-4
Saito K, Hirai T, Takeshima H, Kadota Y, Yamashita S, Ivanova A, Yokogami K (2017) Genetic factors affecting intraoperative 5-aminolevulinic acid-induced fluorescence of diffuse gliomas. Radiol Oncol 51:142–150. https://doi.org/10.1515/raon-2017-0019
Roberts DW, Valdes PA, Harris BT, Fontaine KM, Hartov A, Fan X, Ji S, Lollis SS, Pogue BW, Leblond F, Tosteson TD, Wilson BC, Paulsen KD (2011) Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between delta-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. Clinical article. J Neurosurg 114:595–603. https://doi.org/10.3171/2010.2.JNS091322
Johansson A, Palte G, Schnell O, Tonn JC, Herms J, Stepp H (2010) 5-Aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem Photobiol 86:1373–1378. https://doi.org/10.1111/j.1751-1097.2010.00799.x
Kim A, Khurana M, Moriyama Y, Wilson BC (2010) Quantification of in vivo fluorescence decoupled from the effects of tissue optical properties using fiber-optic spectroscopy measurements. J Biomed Opt. https://doi.org/10.1117/1.3523616
Valdes PA, Leblond F, Kim A, Harris BT, Wilson BC, Fan XY, Tosteson TD, Hartov A, Ji SB, Erkmen K, Simmons NE, Paulsen KD, Roberts DW (2011) Quantitative fluorescence in intracranial tumor: implications for ALA-induced PpIX as an intraoperative biomarker. J Neurosurg 115:11–17. https://doi.org/10.3171/2011.2.Jns101451
Utsuki S, Oka H, Sato S, Suzuki S, Shimizu S, Tanaka S, Fujii K (2006) Possibility of using laser spectroscopy for the intraoperative detection of nonfluorescing brain tumors and the boundaries of brain tumor infiltrates—technical note. J Neurosurg 104:618–620. https://doi.org/10.3171/jns.2006.104.4.618
Valdes PA, Kim A, Brantsch M, Niu C, Moses ZB, Tosteson TD, Wilson BC, Paulsen KD, Roberts DW, Harris BT (2011) Delta-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathologic markers of malignancy in human gliomas: the need for quantitative fluorescence-guided resection to identify regions of increasing malignancy. Neuro-oncology 13:846–856. https://doi.org/10.1093/neuonc/nor086
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chohan, M.O., Berger, M.S. 5-Aminolevulinic acid fluorescence guided surgery for recurrent high-grade gliomas. J Neurooncol 141, 517–522 (2019). https://doi.org/10.1007/s11060-018-2956-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2956-8