Abstract
Purpose
Glioblastoma is the most common primary malignant brain tumor. No standard treatment exists for recurrent disease. Glioblastoma is associated with an immunosuppressive tumor microenvironment. Immune checkpoint inhibitors, including atezolizumab (anti-programmed death-ligand 1), have demonstrated clinical activity in various cancers. Here, we present the safety and efficacy of atezolizumab in patients with glioblastoma from the phase 1a PCD4989g clinical trial (NCT01375842).
Methods
Eligible patients had confirmed recurrent glioblastoma and measurable disease per RANO criteria. Atezolizumab (1200 mg) was administered intravenously every 3 weeks until progression or unacceptable toxicity. Patients were monitored for safety; response was evaluated at least every 6 weeks. Baseline biomarkers were evaluated.
Results
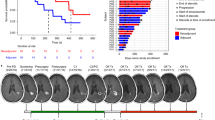
All 16 patients enrolled had received prior chemotherapy, and 50% prior bevacizumab. Ten patients (63%) experienced a treatment-related event. No treatment-related grade 4–5 events were reported. All deaths occurred due to progression or during follow-up. One patient experienced a partial response (5.3 months); 3 experienced disease stabilization. The median overall survival was 4.2 months (range 1.2 to 18.8+ months). Association between peripheral CD4+ T cells and efficacy was observed. Two patients with IDH1-mutant tumors and 1 with a POLE-mutant tumor experienced ≥ 16 months survival.
Conclusions
Atezolizumab was safe and well tolerated in this group of patients with recurrent glioblastoma. Our preliminary findings suggest that biomarkers, including peripheral CD4+ T cells and hypermutated tumor status, may help guide selection of patients with recurrent glioblastoma who might receive most benefit from atezolizumab therapy, supporting further atezolizumab combination studies in glioblastoma.


Similar content being viewed by others
References
Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS (2016) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro Oncol 18:v1–v75. https://doi.org/10.1093/neuonc/now207
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466. https://doi.org/10.1016/S1470-2045(09)70025-7
Lukas RV, Mrugala MM (2017) Pivotal therapeutic trials for infiltrating gliomas and how they affect clinical practice. Neuro-Oncol Pract 4(4):209–216. https://doi.org/10.1093/nop/npw016
Stupp R, Taillibert S, Kanner A, Read W, Steinberg DM, Lhermitte B, Toms S, Idbaih A, Ahluwalia MS, Fink K et al (2017) Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318:2306–2316. https://doi.org/10.1001/jama.2017.18718
Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D, Henriksson R, Balana C et al (2014) EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol 15:e395–e403. https://doi.org/10.1016/S1470-2045(14)70011-7
Touat M, Ibdaih A, Sanson A, Ligon KL (2017) Glioblastoma targeted therapy: updated approaches from recent biological insights. Ann Oncol 28(7):1457–1462. https://doi.org/10.1093/annonc/mdx106
Wong ET, Gautam S, Malchow C, Lun M, Pan E, Brem S (2011) Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw 9:403–407
Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, Kirson ED, Taillibert S, Liebermann F, Dbaly V et al (2012) NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer 48:2192–2202. https://doi.org/10.1016/j.ejca.2012.04.011
Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN et al (2014) Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563–567. https://doi.org/10.1038/nature14011
Chen DS, Irving BA, Hodi FS (2012) Molecular pathways: next-generation immunotherapy: inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 18:6580–6587. https://doi.org/10.1158/1078-0432.CCR-12-1362
Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL et al (2014) MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515:558–562. https://doi.org/10.1038/nature13904
Matsumoto K, Fukuyama S, Eguchi-Tsuda M, Nakano T, Matsumoto T, Matsumura M, Moriwaki A, Kan-o K, Wada Y, Yagita H et al (2008) B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun 365:170–175. https://doi.org/10.1016/j.bbrc.2007.10.156
Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, Sharpe AH, Umetsu DT, Dekruyff RH (2010) PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol 3:81–91. https://doi.org/10.1038/mi.2009.112
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O’Donnell PH, Balmanoukian A, Loriot Y et al (2016) Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 387:1909–1920. https://doi.org/10.1016/S0140-6736(16)00561-4
Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL et al (2017) Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389:67–76
Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC et al (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 389:255–265
Tecentriq (atezolizumab) (2018) [product insert]. Genentech, Inc., South San Francisco
Berghoff AS, Kiesel B, Widhalm G, Rajky O, Ricken G, Wohrer A, Dieckmann K, Filipits M, Brandstetter A, Weller M et al (2015) Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol 17:1064–1075. https://doi.org/10.1093/neuonc/nou307
Vlahovic G, Fecci PE, Reardon D, Sampson JH (2015) Programmed death ligand 1 (PD-L1) as an immunotherapy target in patients with glioblastoma. Neuro Oncol 17:1043–1045. https://doi.org/10.1093/neuonc/nov071
Binder DC, Davis AA, Wainwright DA (2015) Immunotherapy for cancer in the central nervous system: current and future directions. Oncoimmunology 5:e1082027. https://doi.org/10.1080/2162402X.2015.1082027
Balar AV, Weber JS (2017) PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother 66:551–564. https://doi.org/10.1007/s00262-017-1954-6
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509–2520. https://doi.org/10.1056/NEJMoa1500596
McGranahan N, Furness AJ, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak NJ, Hiley CT et al (2016) Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 351:1463–1469. https://doi.org/10.1126/science.aaf1490
Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS et al (2015) Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348:124–128. https://doi.org/10.1126/science.aaa1348
Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, Durno C, Krueger J, Cabric V, Ramaswamy V et al (2016) Immune checkpoint inhibition for hypermutant glioblastoma multiforme resulting from germline biallelic mismatch repair deficiency. J Clin Oncol 34:2206–2211. https://doi.org/10.1200/JCO.2016.66.6552
Hodges TR, Ott M, Xiu J, Gatalica Z, Swensen J, Zhou S, Huse JT, de Groot J, Li S, Overwijk WW et al (2017) Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. https://doi.org/10.1093/neuonc/nox026
Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, Voloschin A, Ramkissoon SH, Ligon KL, Latek R et al (2018) Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase 1 cohorts of CheckMate 143. Neuro Oncol 20:674–686. https://doi.org/10.1093/neuonc/nox208
Reardon DA, Omuro A, Brandes AA, Rieger J, Wick A, Sepulveda J, Phuphanich S, de Souza P, Ahluwalia MS, Lim M et al (2017) Randomized phase 3 study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. WFNOS OS10.3. Neuro-oncology 19(suppl_3):iii21
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. https://doi.org/10.1200/JCO.2009.26.3541
Vennapusa B, Baker B, Kowanetz M, Boone J, Menzl I, Bruey JM, Fine G, Mariathasan S, McCaffery I, Mocci S et al (2018) Development of a PD-L1 Complementary Diagnostic Immunohistochemistry Assay (SP142) for Atezolizumab. Appl Immunohistochem Mol Morphol. https://doi.org/10.1097/PAI.0000000000000594
Chalmers ZR, Connelly CF, Fabrizio D, Gay L, Ali SM, Ennis R, Schrock A, Campbell B, Shlien A, Chmielecki J et al (2017) Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. https://doi.org/10.1186/s13073-017-0424-2
Grant R, Brown PD (2016) Fatigue randomized controlled trials-how tired is “too tired” in patients undergoing glioma treatment? Neuro Oncol 18:759–760. https://doi.org/10.1093/neuonc/now052
Szopa W, Burley TA, Kramer-Marek G, Kaspera W (2017) Diagnostic and therapeutic biomarkers in glioblastoma: current status and future perspectives. Biomed Res Int 2017:8013575. https://doi.org/10.1155/2017/8013575
Palles C, Cazier JB, Howarth KM, Domingo E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I et al (2013) Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet 45:136–144. https://doi.org/10.1038/ng.2503
Johanns TM, Miller CA, Dorward IG, Tsien C, Chang E, Perry A, Uppaluri R, Ferguson C, Schmidt RE, Dahiya S et al (2016) Immunogenomics of hypermutated glioblastoma: a patient with germline POLE deficiency treated with checkpoint blockade Immunotherapy. Cancer Discov 6:1230–1236
Chamberlain MC, Johnston SK (2010) Salvage therapy with single agent bevacizumab for recurrent glioblastoma. J Neurooncol 96:259–269. https://doi.org/10.1007/s11060-009-9957-6
Reardon DA, Desjardins A, Peters KB, Gururangan S, Sampson JH, McLendon RE, Herndon JE 2nd, Bulusu A, Threatt S, Friedman AH et al (2012) Phase II study of carboplatin, irinotecan, and bevacizumab for bevacizumab naive, recurrent glioblastoma. J Neurooncol 107:155–164. https://doi.org/10.1007/s11060-011-0722-2
Huang Y, Goel S, Duda DG, Fukumura D, Jain RK (2013) Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res 73:2943–2948. https://doi.org/10.1158/0008-5472.CAN-12-4354
Nduom EK, Wei J, Yaghi NK, Huang N, Kong LY, Gabrusiewicz K, Ling X, Zhou S, Ivan C, Chen JQ et al (2016) PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol 18:195–205. https://doi.org/10.1093/neuonc/nov172
Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, Richardson W, Towne P, Hanks D, Vennapusa B et al (2017) PD-L1 immunohistochemistry assays for lung cancer: results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol 12:208–222
Seki M, Ushiyama C, Seta N, Abe K, Fukazawa T, Asakawa J, Takasaki Y, Hashimoto H (1998) Apoptosis of lymphocytes induced by glucocorticoids and relationship to therapeutic efficacy in patients with systemic lupus erythematosus. Arthritis Rheum 41:823–830. https://doi.org/10.1002/1529-0131(199805)41:53.0.CO;2-%23
Olnes MJ, Kotliarov Y, Biancotto A, Cheung F, Chen J, Shi R, Zhou H, Wang E, Tsang JS, Nussenblatt R et al (2016) Effects of systemically administered hydrocortisone on the human immunome. Sci Rep 6:23002. https://doi.org/10.1038/srep23002
Grossman SA, Ye X, Lesser G, Sloan A, Carraway H, Desideri S, Piantadosi S, NABTT CNS Consortium (2011) Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res 17:5473–5480. https://doi.org/10.1158/1078-0432.CCR-11-0774
Wong ET, Lok E, Gautam S, Swanson KD (2015) Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer 113:232–241. https://doi.org/10.1038/bjc.2015.238
Waitkus MS, Diplas BH, Yan H (2016) Isocitrate dehydrogenase mutations in gliomas. Neuro Oncol 18:16–26. https://doi.org/10.1093/neuonc/nov136
Kohanbash G, Carrera DA, Shrivastav S, Ahn BJ, Jahan N, Mazor T, Chheda ZS, Downey KM, Watchmaker PB, Beppler C et al (2017) Isocitrate dehydrogenase mutations suppress STAT1 and CD8+ T cell accumulation in gliomas. J Clin Invest 127:1425–1437
Dudley JC, Lin MT, Le DT, Eshleman JR (2016) Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res 22:813–820. https://doi.org/10.1158/1078-0432.CCR-15-1678
Yip S, Miao J, Cahill DP, Iafrate AJ, Aldape K, Nutt CL, Louis DN (2009) MSH6 mutations arise in glioblastomas during temozolomide therapy and mediate temozolomide resistance. Clin Cancer Res 15:4622–4629. https://doi.org/10.1158/1078-0432.CCR-08-3012
van Thuijl HF, Mazor T, Johnson BE, Fouse SD, Aihara K, Hong C, Malmstrom A, Hallbeck M, Heimans JJ, Kloezeman JJ et al (2015) Evolution of DNA repair defects during malignant progression of low-grade gliomas after temozolomide treatment. Acta Neuropathol 129:597–607. https://doi.org/10.1007/s00401-015-1403-6
Howitt BE, Shukla SA, Sholl LM, Ritterhouse LL, Watkins JC, Rodig S, Stover E, Strickland KC, D’Andrea AD, Wu CJ et al (2015) Association of polymerase e-mutated and microsatellite-instable endometrial cancers with neoantigen load, number of tumor-infiltrating lymphocytes, and expression of PD-1 and PD-L1. JAMA Oncol 1:1319–1323. https://doi.org/10.1001/jamaoncol.2015.2151
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A et al (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197. https://doi.org/10.1200/JCO.2007.14.8163
Okada H, Weller M, Huang R, Finocchiaro G, Gilbert MR, Wick W, Ellingson BM, Hashimoto N, Pollack IF, Brandes AA et al (2015) Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol 16:e534–e542. https://doi.org/10.1016/S1470-2045(15)00088-1
Acknowledgements
We thank the patients, their families, and the clinical study site investigators and staff. We also thank Daniel Chen, Gregg Fine, and Cathi Ahearn, of Genentech, Inc., and Helene Cauwel, of Cytel, for their contributions to the study. The authors also thank Dr Roger Stupp for critical review of the manuscript. Medical writing assistance for this manuscript was provided by Minna Balbas, PhD, of Health Interactions, Inc, and funded by F. Hoffmann-La Roche, Ltd. Dr. Lukas is supported by P50CA221747 SPORE for Translational Approaches to Brain Cancer.
Funding
This work was supported by F. Hoffmann-La Roche, Ltd.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RVL has received support from Roche-Genentech for meeting travel to present study results, honoraria for advisory boards for AstraZeneca, Abbvie, and Ziopharm and honoraria for medical editing for EBSCO publishing, Medlink Neurology, and American Physician Institute. JR has received honoraria for advisory boards from Novartis, Lilly, Orion, Servier, and Peptomyc, and has received research funding from Bayer and Novartis. JB has received honoraria for advisory boards from BMS. MF is an employee of, and has stock options with Roche-Genentech, and has stock options and a patent with Aduro BioTech. CO and LM are employees of, and have stock options with Roche-Genentech. SO is an employee of Roche-Genentech. WG, MT, ETW, KS and KB have no conflicts to disclose.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lukas, R.V., Rodon, J., Becker, K. et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J Neurooncol 140, 317–328 (2018). https://doi.org/10.1007/s11060-018-2955-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2955-9