Abstract
Background
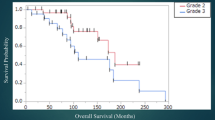
Lower-grade gliomas (LGGs, defined as WHO grades II and III) with 1p19q codeletion have increased chemosensitivity when compared to LGGs without 1p19q codeletion, but the mechanism is currently unknown.
Methods
RNAseq data from 515 LGG patients in the Cancer Genome Atlas (TCGA) were analyzed to compare the effect of expression of the 9 DNA repair genes located on chromosome arms 1p and 19q on progression free survival (PFS) and overall survival (OS) between patients who received chemotherapy and those who did not. Chemosensitivity of cells with DNA repair genes knocked down was tested using MTS cell proliferation assay in HS683 cell line and U251 cell line.
Results
The expression of 9 DNA repair genes on 1p and 19q was significantly lower in 1p19q-codeleted tumors (n = 175) than in tumors without the codeletion (n = 337) (p < 0.001). In LGG patients who received chemotherapy, lower expression of LIG1, POLD1, PNKP, RAD54L and MUTYH was associated with longer PFS and OS. This difference between chemotherapy and non-chemotherapy groups in the association of gene expression with survival was not observed in non-DNA repair genes located on chromosome arms 1p and 19q. MTS assays showed that knockdown of DNA repair genes LIG1, POLD1, PNKP, RAD54L and MUTYH significantly inhibited recovery in response to temozolomide when compared with control group (p < 0.001).
Conclusions
Our results suggest that reduced expression of DNA repair genes on chromosome arms 1p and 19q may account for the increased chemosensitivity of LGGs with 1p19q codeletion.




Similar content being viewed by others
References
Dolecek TA, Propp JM, Stroup NE, Kruchko C (2012) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro-oncology 14 Suppl(5):v1–v49. https://doi.org/10.1093/neuonc/nos218
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1
Cancer Genome Atlas Research N, Brat DJ, Verhaak RG et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372 (26):2481–2498. https://doi.org/10.1056/NEJMoa1402121
Shaw EG, Wang M, Coons SW et al (2012) Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol 30(25):3065–3070. https://doi.org/10.1200/JCO.2011.35.8598
Buckner JC, Shaw EG, Pugh SL et al (2016) Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med 374(14):1344–1355. https://doi.org/10.1056/NEJMoa1500925
Eckel-Passow JE, Lachance DH, Molinaro AM et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372(26):2499–2508. https://doi.org/10.1056/NEJMoa1407279
Cairncross G, Wang M, Shaw E et al (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31(3):337–343. https://doi.org/10.1200/JCO.2012.43.2674
van den Bent MJ, Brandes AA, Taphoorn MJ et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31(3):344–350. https://doi.org/10.1200/JCO.2012.43.2229
Boccard SG, Marand SV, Geraci S, Pycroft L, Berger FR, Pelletier LA (2015) Inhibition of DNA-repair genes Ercc1 and Mgmt enhances temozolomide efficacy in gliomas treatment: a pre-clinical study. Oncotarget 6(30):29456–29468. https://doi.org/10.18632/oncotarget.4909
Wood RD, Mitchell M, Lindahl T (2005) Human DNA repair genes. Mutat Res 577(1–2):275–283. https://doi.org/10.1016/j.mrfmmm.2005.03.007
Kamoun A, Idbaih A, Dehais C et al (2016) Integrated multi-omics analysis of oligodendroglial tumours identifies three subgroups of 1p/19q co-deleted gliomas. Nat Commun 7:11263. https://doi.org/10.1038/ncomms11263
Wang S, Jin F, Fan W et al (2017) Gene expression meta-analysis in diffuse low-grade glioma and the corresponding histological subtypes. Sci Rep 7(1):11741. https://doi.org/10.1038/s41598-017-12087-y
Zhou Q, Guo P, Kruh GD, Vicini P, Wang X, Gallo JM (2007) Predicting human tumor drug concentrations from a preclinical pharmacokinetic model of temozolomide brain disposition. Clin Cancer Res 13(14):4271–4279. https://doi.org/10.1158/1078-0432.CCR-07-0658
Jenkins RB, Blair H, Ballman KV et al (2006) A t(1;19)(q10;p10) mediates the combined deletions of 1p and 19q and predicts a better prognosis of patients with oligodendroglioma. Cancer Res 66(20):9852–9861. https://doi.org/10.1158/0008-5472.CAN-06-1796
Jackson SP, Helleday T (2016) DNA repair. drugging DNA repair. Science 352(6290):1178–1179. https://doi.org/10.1126/science.aab0958
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352(10):997–1003. https://doi.org/10.1056/NEJMoa043331
Viel T, Monfared P, Schelhaas S et al (2013) Optimizing glioblastoma temozolomide chemotherapy employing lentiviral-based anti-MGMT shRNA technology. Mol Ther 21(3):570–579. https://doi.org/10.1038/mt.2012.278
Short SC, Giampieri S, Worku M et al (2011) Rad51 inhibition is an effective means of targeting DNA repair in glioma models and CD133 + tumor-derived cells. Neuro-oncology 13(5):487–499. https://doi.org/10.1093/neuonc/nor010
Kitange GJ, Mladek AC, Schroeder MA et al (2016) Retinoblastoma binding protein 4 modulates temozolomide sensitivity in glioblastoma by regulating DNA repair proteins. Cell Rep 14(11):2587–2598. https://doi.org/10.1016/j.celrep.2016.02.045
McFaline-Figueroa JL, Braun CJ, Stanciu M et al (2015) Minor changes in expression of the mismatch repair protein MSH2 exert a major impact on glioblastoma response to temozolomide. Cancer Res 75(15):3127–3138. https://doi.org/10.1158/0008-5472.CAN-14-3616
Dang L, White DW, Gross S et al (2009) Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462(7274):739–744. https://doi.org/10.1038/nature08617
Sulkowski PL, Corso CD, Robinson ND et al (2017) 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med 9(375):eaal2463. https://doi.org/10.1126/scitranslmed.aal2463
Poulsen ML, Bisgaard ML (2008) MUTYH associated polyposis (MAP). Curr Genomics 9(6):420–435. https://doi.org/10.2174/138920208785699562
Annovazzi L, Mellai M, Schiffer D (2017) Chemotherapeutic drugs: DNA damage and repair in glioblastoma. Cancers 9(6):57. https://doi.org/10.3390/cancers9060057
Liang BC, Ross DA, Reed E (1995) Genomic copy number changes of DNA repair genes ERCC1 and ERCC2 in human gliomas. J Neuro-Oncol 26(1):17–23
Chen HY, Shao CJ, Chen FR, Kwan AL, Chen ZP (2010) Role of ERCC1 promoter hypermethylation in drug resistance to cisplatin in human gliomas. Int J Cancer 126(8):1944–1954. https://doi.org/10.1002/ijc.24772
Vago R, Leva V, Biamonti G, Montecucco A (2009) DNA ligase I and Nbs1 proteins associate in a complex and colocalize at replication factories. Cell Cycle 8(16):2600–2607. https://doi.org/10.4161/cc.8.16.9352
Sun D, Urrabaz R, Buzello C, Nguyen M (2002) Effects of cisplatin on expression of DNA ligases in MiaPaCa human pancreatic cancer cells. Biochem Biophys Res Commun 298(4):537–544
Barnes DE, Tomkinson AE, Lehmann AR, Webster AD, Lindahl T (1992) Mutations in the DNA ligase I gene of an individual with immunodeficiencies and cellular hypersensitivity to DNA-damaging agents. Cell 69(3):495–503
Prindle MJ, Loeb LA (2012) DNA polymerase delta in DNA replication and genome maintenance. Environ Mol Mutagen 53(9):666–682. https://doi.org/10.1002/em.21745
Hocke S, Guo Y, Job A et al (2016) A synthetic lethal screen identifies ATR-inhibition as a novel therapeutic approach for POLD1-deficient cancers. Oncotarget 7(6):7080–7095. https://doi.org/10.18632/oncotarget.6857
Mazin AV, Mazina OM, Bugreev DV, Rossi MJ (2010) Rad54, the motor of homologous recombination. DNA Repair 9(3):286–302. https://doi.org/10.1016/j.dnarep.2009.12.006
Mahabir AG, Schaap M, Theunissen P et al (2008) DNA-repair-deficient Rad54/Rad54B mice are more sensitive to clastogens than wild-type mice. Toxicol Lett 183(1–3):112–117. https://doi.org/10.1016/j.toxlet.2008.10.005
Ghamrasni SE, Cardoso R, Li L et al (2016) Rad54 and Mus81 cooperation promotes DNA damage repair and restrains chromosome missegregation. Oncogene 35(37):4836–4845. https://doi.org/10.1038/onc.2016.16
Brandes AA, Nicolardi L, Tosoni A et al (2006) Survival following adjuvant PCV or temozolomide for anaplastic astrocytoma. Neuro-Oncol 8(3):253–260. https://doi.org/10.1215/15228517-2006-005
Acknowledgements
This study was supported by Shenghua Yuying Project of Central South University to L.Y., National Science Foundation of China to XJL (81472594 and 81770781), and National Science and Technology Major Project to B.X. (2016YFC0904400).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2018_2915_MOESM1_ESM.tif
Supplementary Figure 1. The expression of 5 DNA repair genes on chromosome arms 1q and 19p in patients with versus without 1p19q codeletion. (TIF 128 KB)
11060_2018_2915_MOESM2_ESM.tif
Supplementary Figure 2. The expression of 5 most upregulated and 5 most downregulated non-DNA repair genes on chromosome arms 1p and 19q relative to non-glioma sample in patients with versus without 1p19q codeletion. (TIF 1377 KB)
11060_2018_2915_MOESM3_ESM.tif
Supplementary Figure 3. Progression free survival of patients who received chemotherapy between low expression and high expression for (A) LIG1, (B) POLD1, (C) PNKP, (D) RAD54L, (E) MUTYH. (TIF 1463 KB)
11060_2018_2915_MOESM4_ESM.tif
Supplementary Figure 4. Overall survival of patients who received chemotherapy between low expression and high expression for (A) LIG1, (B) POLD1, (C) PNKP, (D) RAD54L, (E) MUTYH. (TIF 1488 KB)
11060_2018_2915_MOESM5_ESM.tif
Supplementary Figure 5. Results of RT-qPCR of HS683 cell line demonstrate good knockdown efficiency for shRAD54L#1, shRAD54L#2, shRAD54L#3, shMUTYH#1, shMUTYH#2, shMUTYH#3, shLIG1#1, shLIG1#2, shLIG1#3, shPNKP#1, shPNKP#2, shPNKP#3, shPOLD1#1 and shPOLD1#2. (TIF 276 KB)
11060_2018_2915_MOESM6_ESM.tif
Supplementary Figure 6. Results of RT-qPCR of U251 cell line demonstrate good knockdown efficiency for shRAD54L#1, shRAD54L#2, shRAD54L#3, shMUTYH#1, shMUTYH#2, shMUTYH#3, shLIG1#1, shLIG1#2, shLIG1#3, shPNKP#1, shPNKP#2, shPNKP#3, shPOLD1#1 and shPOLD1#2. (TIF 265 KB)
11060_2018_2915_MOESM7_ESM.tif
Supplementary Figure 7. MTS curve after knockdown sequence of GFP for: (A) HS683-2mg/L TMZ, (B) U251-2mg/L TMZ, (C) HS683-5mg/L TMZ, (D) U251-5mg/L TMZ, (E) HS683-10mg/L TMZ, (F) U251-10mg/L TMZ. (TIF 201 KB)
11060_2018_2915_MOESM8_ESM.tif
Supplementary Figure 8. MTS curve of HS683 cell line at 5mg/L for: (A) RAD54L, (B) MUTYH, (C) LIG1, (D) PNKP, (E) POLD1. *, p<0.05; **, p<0.01; ***, p<0.001. (TIF 221 KB)
11060_2018_2915_MOESM9_ESM.tif
Supplementary Figure 9. MTS curve of U251 cell line at 5mg/L for: (A) RAD54L, (B) MUTYH, (C) LIG1, (D) PNKP, (E) POLD1. *, p<0.05; **, p<0.01; ***, p<0.001. (TIF 215 KB)
11060_2018_2915_MOESM10_ESM.tif
Supplementary Figure 10. MTS curve of HS683 cell line at 10mg/L for: (A) RAD54L, (B) MUTYH, (C) LIG1, (D) PNKP, (E) POLD1. *, p<0.05; **, p<0.01; ***, p<0.001. (TIF 220 KB)
11060_2018_2915_MOESM11_ESM.tif
Supplementary Figure 11. MTS curve of U251 cell line at 10mg/L for: (A) RAD54L, (B) MUTYH, (C) LIG1, (D) PNKP, (E) POLD1. *, p<0.05; **, p<0.01; ***, p<0.001. (TIF 216 KB)
Rights and permissions
About this article
Cite this article
Tang, L., Deng, L., Bai, H.X. et al. Reduced expression of DNA repair genes and chemosensitivity in 1p19q codeleted lower-grade gliomas. J Neurooncol 139, 563–571 (2018). https://doi.org/10.1007/s11060-018-2915-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-2915-4