Abstract
Introduction
Differentiation of normal pituitary from abnormal tumor tissue remains a surgical challenge despite improvements in optical visualization technology for pituitary adenoma (PA) surgery. During neurosurgical procedures for other tumor types, 5-aminolevulinic acid (5-ALA) has become a focus of investigation based on its high specificity in differentiating tumor tissue. However, the role of 5-ALA and other optical fluorescent agents in PA surgery remains less clear.
Objective
To perform a systematic review on the use of various optical fluorescent agents in PA surgery.
Method
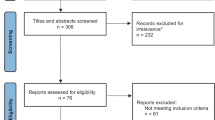
Using PRISMA guidelines, a systematic literature review to identify reports describing 5-ALA and other optical agents for fluorescence-guided surgery for PA was performed. Eleven research studies met inclusion criteria and were reviewed.
Results
In two studies, 5-ALA was not shown to be effective in aiding PA resection using standard neurosurgical endoscopic/microscopic approaches. 5-ALA photodynamic therapy was evaluated in two in-vitro models with inconsistent results. Intraoperative use of indocyanine green (ICG) concluded with varying results, but showed a tendency towards improved differentiation of functional PA. OTL38 showed potential for intraoperative identification of nonfunctioning PA, particularly in tumors with high folate receptor expression. One study reported clinically useful fluorescence following sodium fluorescein administration.
Conclusion
We conclude that selected optical fluorescent agents, including ICG and folate receptors, are most likely to hold promise for clinical use in differentiating PA from normal tissue.
Similar content being viewed by others
References
Melmed S (2011) Pathogenesis of pituitary tumors. Nat Rev Endocrinol 7:257–266. https://doi.org/10.1038/nrendo.2011.40
Kovacs K, Horvath E, Vidal S (2001) Classification of pituitary adenomas. J Neurooncol 54:121–127. https://doi.org/10.1023/A:1012945129981
Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A (2006) High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 91:4769–4775. https://doi.org/10.1210/jc.2006-1668
Dusek T, Kastelan D, Melada A, Baretic M, Skoric Polovina T, Perkovic Z, Giljevic Z, Jelcic J, Paladino J, Korsic M, Aganovic I (2011) Clinical features and therapeutic outcomes of patients with acromegaly: single-center experience. Endocrinol Invest 32:e382–e385. https://doi.org/10.3275/7858
Juszczak A, Ertorer ME, Grossman A (2013) The therapy of Cushing’s disease in adults and children: an update. Horm Metab Res 45:109–117. https://doi.org/10.1055/s-0032-1330009
Krzentowska-Korek A, Gołkowski F, Bałdys-Waligórska A, Hubalewska-Dydejczyk A (2011) Efficacy and complications of neurosurgical treatment of acromegaly. Pituitary 14:157–162. https://doi.org/10.1007/s11102-010-0273-0
Berkmann S, Schlaffer S, Nimsky C, Fahlbusch R, Buchfelder M (2014) Follow-up and long-term outcome of nonfunctioning pituitary adenoma operated by transsphenoidal surgery with intraoperative high-field magnetic resonance imaging. Acta Neurochir 156:2233–2243. https://doi.org/10.1007/s00701-014-2210-x
Stummer W, Reulen HJ, Meinel T, Pichlmeier U, Schumacher W, Tonn JC, Rohde V, Oppel F, Turowski B, Woiciechowsky C, Franz K, Pietsch T (2008) Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery 62:564–576. https://doi.org/10.1227/01.neu.0000317304.31579.17
Biller BMK, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JAH, Boscaro M (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93:2454–2462
Jane JA Jr., Laws ER Jr. (2000) Surgical treatment of pituitary adenomas BTI-Endotext. In: De Groot LJCG, Dungan K et al (ed) MDText.com, Inc., South Dartmouth
Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, Wass JA (2011) Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:273–288. https://doi.org/10.1210/jc.2010-1692
Giustina A, Chanson P, Kleinberg D, Bronstein MD, Clemmons DR, Klibanski A, van der Lely AJ, Strasburger CJ, Lamberts SW, Ho KK, Casanueva FF, Melmed S (2014) Expert consensus document: a consensus on the medical treatment of acromegaly. Nat Rev Endocrinol 10:243. https://doi.org/10.1038/nrendo.2014.21
Carr SB, Kleinschmidt-DeMasters BK, Kerr JM, Kiseljak-Vassiliades K, Wierman ME, Lillehei KO (2017) Negative surgical exploration in patients with Cushing’s disease: benefit of two-thirds gland resection on remission rate and a review of the literature. J Neurosurg 8:1–8. https://doi.org/10.3171/2017.5.JNS162901
Ausiello JC, Bruce JN, Freda PU (2008) Postoperative assessment of the patient after transsphenoidal pituitary surgery. Pituitary 11:391–401. https://doi.org/10.1007/s11102-008-0086-6
Bellut D, Hlavica M, Muroi C, Woernle CM, Schmid C, Bernays RL (2012) Impact of intraoperative MRI-guided transsphenoidal surgery on endocrine function and hormone substitution therapy in patients with pituitary adenoma. Swiss Med Wkly 142:w13699. https://doi.org/10.4414/smw.2012.13699
Fomekong E, Duprez T, Docquier MA, Ntsambi G, Maiter D, Raftopoulos C (2014) Intraoperative 3T MRI for pituitary macroadenoma resection: initial experience in 73 consecutive patients. Clin Neurol Neurosurg 126:143–149. https://doi.org/10.1016/j.clineuro.2014.09.001
Paterno V, Fahlbusch R (2014) High-field iMRI in transsphenoidal pituitary adenoma surgery with special respect to typical localization of residual tumor. Acta Neurochir (Wien) 156:463–474. https://doi.org/10.1007/s00701-013-1978-4 (discussion 474)
Semple PL, Vance ML, Findling J, Laws ER Jr. (2000) Transsphenoidal surgery for Cushing’s disease: outcome in patients with a normal magnetic resonance imaging scan. Neurosurgery 46:553–558
Abe T, Matsumoto K, Kuwazawa J, Toyoda I, Sasaki K (1998) Headache associated with pituitary adenomas. Headache 38:782–786
Bengtsson BA, Eden S, Ernest I, Oden A, Sjogren B (1988) Epidemiology and long-term survival in acromegaly. A study of 166 cases diagnosed between 1955 and 1984. Acta Med Scand 223:327–335
Etxabe J, Vazquez JA (1994) Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf) 40:479–484
Holdaway IM, Bolland MJ, Gamble GD (2008) A meta-analysis of the effect of lowering serum levels of GH and IGF-I on mortality in acromegaly. Eur J Endocrinol 158:89–95. https://doi.org/10.1530/EJE-08-0267
Newell-Price J, Bertagna X, Grossman AB, Nieman LK (2006) Cushing’s syndrome. Lancet 367:1605–1617. https://doi.org/10.1016/S0140-6736(06)68699-6
Nomikos P, Buchfelder M, Fahlbusch R (2005) The outcome of surgery in 668 patients with acromegaly using current criteria of biochemical ‘cure’. Eur J Endocrinol 152:379–387
Raverot G, Burman P, McCormack A, Heaney A, Petersenn S, Popovic V, Trouillas J, Dekkers OM (2018) European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol 178:G1–G24. https://doi.org/10.1530/EJE-17-0796
Sughrue ME, Blevins LS, Chang EF, Gabriel RA, Aghi MK, Blevins LS (2011) Excess mortality for patients with residual disease following resection of pituitary adenomas. Pituitary 14:276–283. https://doi.org/10.1007/s11102-011-0308-1
Hansen TM, Batra S, Lim M, Gallia GL, Burger PC, Salvatori R, Wand G, Quinones-Hinojosa A, Kleinberg L, Redmond KJ (2014) Invasive adenoma and pituitary carcinoma: a SEER database analysis. Neurosurg Rev 37:279–286. https://doi.org/10.1007/s10143-014-0525-y
Alander JT, Kaartinen I, Laakso A, Patila T, Spillmann T, Tuchin VV, Venermo M, Valisuo P (2012) A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012:7. https://doi.org/10.1155/2012/940585
Hide T, Yano S, Shinojima N, Kuratsu J (2015) Usefulness of the indocyanine green fluorescence endoscope in endonasal transsphenoidal surgery. J Neurosurg 122:1185–1192. https://doi.org/10.3171/2014.9.JNS14599
Verstegen MJT, Tummers QRJG, Schutte PJ, Pereira AM, van Furth WR, van de Velde CJH, Malessy MJA, Vahrmeijer AL (2016) Intraoperative identification of a normal pituitary gland and an adenoma using near-infrared fluorescence imaging and low-dose indocyanine green. Oper Neurosurg 12:260–268. https://doi.org/10.1227/NEU.0000000000001328
Cho SS, Zeh R, Pierce JT, Jeon J, Nasrallah M, Adappa ND, Palmer JN, Newman JG, White C, Kharlip J, Snyder P, Low P, Singhal S, Grady MS, Lee JYK (2018) Folate receptor near-infrared optical imaging provides sensitive and specific intraoperative visualization of nonfunctional pituitary adenomas. Oper Neurosurg. https://doi.org/10.1093/ons/opy034
Lee JYK, Cho SS, Zeh R, Pierce JT, Martinez-Lage M, Adappa ND, Palmer JN, Newman JG, Learned KO, White C, Kharlip J, Snyder P, Low PS, Singhal S, Grady MS (2018) Folate receptor overexpression can be visualized in real time during pituitary adenoma endoscopic transsphenoidal surgery with near-infrared imaging. J Neurosurg 129:390–403. https://doi.org/10.3171/2017.2.JNS163191
Shinoda J, Yano H, Yoshimura S, Okumura A, Kaku Y, Iwama T, Sakai N (2003) Fluorescence-guided resection of glioblastoma multiforme by using high-dose fluorescein sodium. Technical note. J Neurosurg 99:597–603
Zhang DY, Singhal S, Lee JYK (2018) Optical principles of fluorescence-guided brain tumor surgery: a practical primer for the neurosurgeon. Neurosurgery. https://doi.org/10.1093/neuros/nyy315
Coburger J, Hagel V, Wirtz CR, König R (2015) Surgery for glioblastoma: impact of the combined use of 5-aminolevulinic acid and intraoperative MRI on extent of resection and survival. PLoS ONE 10:e0131872. https://doi.org/10.1371/journal.pone.0131872
Marbacher S, Klinger E, Schwyzer L, Fischer I, Nevzati E, Diepers M, Roelcke U, Fathi AR, Coluccia D, Fandino J (2014) Use of fluorescence to guide resection or biopsy of primary brain tumors and brain metastases. Neurosurg Focus 36:E10. https://doi.org/10.3171/2013.12.FOCUS13464
Hadjipanayis CG, Widhalm G, Stummer W (2015) What is the surgical benefit of utilizing 5-ALA for fluorescence-guided surgery of malignant gliomas? Neurosurgery 77:663–673. https://doi.org/10.1227/NEU.0000000000000929
Haj-Hosseini N, Richter JCO, Hallbeck M, Wårdell K (2015) Low dose 5-aminolevulinic acid: implications in spectroscopic measurements during brain tumor surgery. Photodiagn Photodyn Ther 12:209–214. https://doi.org/10.1016/j.pdpdt.2015.03.004
Kennedy JC, Pottier RH (1992) Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J Photochem Photobiol 14:275–292
Moher D, Liberati A, Tetzlaff J, Altman DG, The PG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLOS Med 6:e1000097
Eljamel MS, Leese G, Moseley H (2009) Intraoperative optical identification of pituitary adenomas. J Neurooncol 92:417–421. https://doi.org/10.1007/s11060-009-9820-9
Nemes A, Fortmann T, Poeschke S, Greve B, Prevedello D, Santacroce A, Stummer W, Senner V, Ewelt C (2016) 5-ALA fluorescence in native pituitary adenoma cell lines: resection control and basis for photodynamic therapy (PDT)? PLoS ONE 11:e0161364. https://doi.org/10.1371/journal.pone.0161364
Neumann LM, Beseoglu K, Slotty PJ, Senger B, Kamp MA, Hänggi D, Steiger HJ, Cornelius JF (2016) Efficacy of 5-aminolevulinic acid based photodynamic therapy in pituitary adenomas-experimental study on rat and human cell cultures. Photodiagn Photodyn Ther 14:77–83. https://doi.org/10.1016/j.pdpdt.2016.02.006
Litvack ZN, Zada G, Laws ER (2012) Indocyanine green fluorescence endoscopy for visual differentiation of pituitary tumor from surrounding structures. J Neurosurg 116:935–941. https://doi.org/10.3171/2012.1.JNS11601
Sandow N, Klene W, Elbelt U, Strasburger CJ, Vajkoczy P (2015) Intraoperative indocyanine green videoangiography for identification of pituitary adenomas using a microscopic transsphenoidal approach. Pituitary 18:613–620. https://doi.org/10.1007/s11102-014-0620-7
da Silva CE, da Silva JL, da Silva VD (2010) Use of sodium fluorescein in skull base tumors. Surg Neurol Int 1:70. https://doi.org/10.4103/2152-7806.72247
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Stephanie Wan-Ting Chang declares that she has no conflict of interest. Author Daniel A. Donoho declares that he has no conflict of interest. Author Gabriel Zada declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chang, S.W., Donoho, D.A. & Zada, G. Use of optical fluorescence agents during surgery for pituitary adenomas: current state of the field. J Neurooncol 141, 585–593 (2019). https://doi.org/10.1007/s11060-018-03062-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03062-2