Abstract
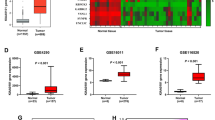
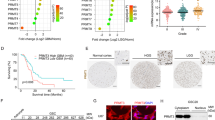
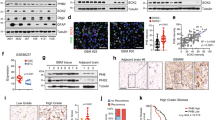
Romo1 is a mitochondrial protein whose elevated expression is commonly observed in various types of human cancers. However, the expression status of Romo1 and its implication in the pathogenesis of human glioblastoma (GBM) remain largely undefined. To understand the role of Romo1 in the progression of GBM, we explored its expression in a series of GBM tissues and cell lines and determined its effect on ROS production, cell proliferation, and tumor growth. Romo1 was frequently overexpressed at the mRNA level in both primary tumors and cell lines and its elevation was more commonly observed in high grade tumors versus low grade tumors. Romo1 expression was associated with ROS production and its knockdown led to a marked reduction of in vitro cellular growth and anchorage-independent growth of GBM. Consistently, Romo1 depletion induced a G2/M arrest of the cell cycle that was accompanied with accumulation of phospho-cdc2. Furthermore, a mouse xenograft assay revealed that Romo1 depletion significantly decreased tumor formation and growth. Therefore, our data demonstrate that Romo1 upregulation is a common event in human GBMs and contributes to the malignant tumor progression, suggesting that Romo1 could be a new therapeutic target for human GBM.




Similar content being viewed by others
References
Chamberlain M (2011) Evolving strategies: future treatment of glioblastoma. Expert Rev Neurother 11:519–532. doi:10.1586/ern.11.30
Henriksson R, Asklund T, Poulsen HS (2011) Impact of therapy on quality of life, neurocognitive function and their correlates in glioblastoma multiforme: a review. J Neurooncol 104:639–646. doi:10.1007/s11060-011-0565-x
Wheeler CJ, Black KL (2011) Vaccines for glioblastoma and high-grade glioma. Expert rev vaccines 10:875–886. doi:10.1586/erv.11.71
(2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455: 1061–1068 doi: 10.1038/nature07385
Piccolo SR, Frey LJ (2013) Clinical and molecular models of glioblastoma multiforme survival. Int J Data mining bioinform 7:245–265
Clarke J, Penas C, Pastori C, Komotar RJ, Bregy A, Shah AH, Wahlestedt C, Ayad NG (2013) Epigenetic pathways and glioblastoma treatment. Epigenetics 8:785–795
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD (2009) IDH1 and IDH2 mutations in gliomas. New Engl J Med 360:765–773. doi:10.1056/NEJMoa0808710
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O’Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, Hodgson JG, James CD, Sarkaria JN, Brennan C, Kahn A, Spellman PT, Wilson RK, Speed TP, Gray JW, Meyerson M, Getz G, Perou CM, Hayes DN (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110. doi:10.1016/j.ccr.2009.12.020
Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC (2010) Metabolic modulation of glioblastoma with dichloroacetate. Sci Transl Med 2:31ra34. doi:10.1126/scitranslmed.3000677
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279:L1005–L1028
Mittler R, Vanderauwera S, Suzuki N, Miller G, Tognetti VB, Vandepoele K, Gollery M, Shulaev V, Van Breusegem F (2011) ROS signaling: the new wave? Trends Plant Sci 16:300–309. doi:10.1016/j.tplants.2011.03.007
Hamanaka RB, Chandel NS (2010) Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci 35:505–513
D’Autreaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8:813–824. doi:10.1038/nrm2256
Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P (2008) Redox regulation of cell survival. Antioxid Redox Signal 10:1343–1374. doi:10.1089/ars.2007.1957
Dewaele M, Maes H, Agostinis P (2010) ROS-mediated mechanisms of autophagy stimulation and their relevance in cancer therapy. Autophagy 6:838–854
Trachootham D, Alexandre J, Huang P (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8:579–591. doi:10.1038/nrd2803
Pelicano H, Carney D, Huang P (2004) ROS stress in cancer cells and therapeutic implications. Drug Resist Updates 7:97–110. doi:10.1016/j.drup.2004.01.004
Silber JR, Bobola MS, Blank A, Schoeler KD, Haroldson PD, Huynh MB, Kolstoe DD (2002) The apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clin Cancer Res 8:3008–3018
Chung YM, Kim JS, Yoo YD (2006) A novel protein, Romo1, induces ROS production in the mitochondria. Biochem Biophys Res Comm 347:649–655. doi:10.1016/j.bbrc.2006.06.140
Chung YM, Lee SB, Kim HJ, Park SH, Kim JJ, Chung JS, Yoo YD (2008) Replicative senescence induced by Romo1-derived reactive oxygen species. J Biol Chem 283:33763–33771. doi:10.1074/jbc.M805334200
Hwang IT, Chung YM, Kim JJ, Chung JS, Kim BS, Kim HJ, Kim JS, Yoo YD (2007) Drug resistance to 5-FU linked to reactive oxygen species modulator 1. Biochem Biophys Res Comm 359:304–310. doi:10.1016/j.bbrc.2007.05.088
Na AR, Chung YM, Lee SB, Park SH, Lee MS, Yoo YD (2008) A critical role for Romo1-derived ROS in cell proliferation. Biochem Biophys Res Comm 369:672–678. doi:10.1016/j.bbrc.2008.02.098
Lee SB, Kim JJ, Kim TW, Kim BS, Lee MS, Yoo YD (2010) Serum deprivation-induced reactive oxygen species production is mediated by Romo1. Apoptosis Int J Progr Cell Death 15:204–218. doi:10.1007/s10495-009-0411-1
Kim JJ, Lee SB, Park JK, Yoo YD (2010) TNF-alpha-induced ROS production triggering apoptosis is directly linked to Romo1 and Bcl-X(L). Cell Death Differ 17:1420–1434. doi:10.1038/cdd.2010.19
Chung JS, Park S, Park SH, Park ER, Cha PH, Kim BY, Chung YM, Woo SR, Han CJ, Kim SB, Suh KS, Jang JJ, Lee K, Choi DW, Lee S, Lee GY, Hahm KB, Shin JA, Kim BS, Noh KH, Kim TW, Lee KH, Yoo YD (2012) Overexpression of Romo1 promotes production of reactive oxygen species and invasiveness of hepatic tumor cells. Gastroenterology 143(1084–1094):e1087. doi:10.1053/j.gastro.2012.06.038
Liu M, Dai B, Kang SH, Ban K, Huang FJ, Lang FF, Aldape KD, Xie TX, Pelloski CE, Xie K, Sawaya R, Huang S (2006) FoxM1B is overexpressed in human glioblastomas and critically regulates the tumorigenicity of glioma cells. Cancer Res 66:3593–3602. doi:10.1158/0008-5472.can-05-2912
Liou GY, Storz P (2010) Reactive oxygen species in cancer. Free Radical Res 44:479–496. doi:10.3109/10715761003667554
Iida T, Furuta A, Kawashima M, Nishida J, Nakabeppu Y, Iwaki T (2001) Accumulation of 8-oxo-2’-deoxyguanosine and increased expression of hMTH1 protein in brain tumors. Neuro-oncol 3:73–81
Cobbs CS, Brenman JE, Aldape KD, Bredt DS, Israel MA (1995) Expression of nitric oxide synthase in human central nervous system tumors. Cancer Res 55:727–730
Yoshii Y, Saito A, Zhao DW, Nose T (1999) Copper/zinc superoxide dismutase, nuclear DNA content, and progression in human gliomas. J Neurooncol 42:103–108
Nitta M, Kozono D, Kennedy R, Stommel J, Ng K, Zinn PO, Kushwaha D, Kesari S, Inda MD, Wykosky J, Furnari F, Hoadley KA, Chin L, DePinho RA, Cavenee WK, D’Andrea A, Chen CC (2010) Targeting EGFR induced oxidative stress by PARP1 inhibition in glioblastoma therapy. PLoS ONE 5:e10767. doi:10.1371/journal.pone.0010767
Zhao J, Liu T, Jin SB, Tomilin N, Castro J, Shupliakov O, Lendahl U, Nister M (2009) The novel conserved mitochondrial inner-membrane protein MTGM regulates mitochondrial morphology and cell proliferation. J Cell Sci 122:2252–2262. doi:10.1242/jcs.038513
Fulda S, Galluzzi L, Kroemer G (2010) Targeting mitochondria for cancer therapy. Nat Rev Drug Discov 9:447–464. doi:10.1038/nrd3137
Storz P (2005) Reactive oxygen species in tumor progression. Front Biosci 10:1881–1896
Szatrowski TP, Nathan CF (1991) Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res 51:794–798
Factor VM, Laskowska D, Jensen MR, Woitach JT, Popescu NC, Thorgeirsson SS (2000) Vitamin E reduces chromosomal damage and inhibits hepatic tumor formation in a transgenic mouse model. Proc Natl Acad Sci USA 97:2196–2201. doi:10.1073/pnas.040428797
Russ WP, Engelman DM (2000) The GxxxG motif: a framework for transmembrane helix–helix association. J Mol Biol 296:911–919. doi:10.1006/jmbi.1999.3489
Chung JS, Lee SB, Park SH, Kang ST, Na AR, Chang TS, Kim HJ, Yoo YD (2009) Mitochondrial reactive oxygen species originating from Romo1 exert an important role in normal cell cycle progression by regulating p27(Kip1) expression. Free Radical Res 43:729–737. doi:10.1080/10715760903038432
Mori S, Chang JT, Andrechek ER, Matsumura N, Baba T, Yao G, Kim JW, Gatza M, Murphy S, Nevins JR (2009) Anchorage-independent cell growth signature identifies tumors with metastatic potential. Oncogene 28:2796–2805. doi:10.1038/onc.2009.139
Hamburger A, Lurie K, Condon M (1987) Stimulation of anchorage-independent growth of human tumor cells by interleukin 1. Cancer Res 47:5612–5615
Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK (2008) Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68:1777–1785
Venkataraman S, Jiang X, Weydert C, Zhang Y, Zhang HJ, Goswami PC, Ritchie JM, Oberley LW, Buettner GR (2004) Manganese superoxide dismutase overexpression inhibits the growth of androgen-independent prostate cancer cells. Oncogene 24:77–89
Acknowledgments
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2010-0009959).
Conflict of interest
None.
Ethical standards
The animal experiment was approved by Committees at the Korea university and tissue samples were collected after obtain patients’ informed consent.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2014_1608_MOESM1_ESM.docx
Validation of knockdown effect of lentiviral transfection of Romo1 shRNAs. a Comparison of knockdown effect of four different shRNAs. GBM cells were infected with lentivirus containing control shRNA and four different Romo1 shRNAs (1-4). At 24 h after infection, Romo1 levels were determined. b Knockdown effect of shRNA-1 on Romo1 expression
Rights and permissions
About this article
Cite this article
Yu, M.O., Song, NH., Park, KJ. et al. Romo1 is associated with ROS production and cellular growth in human gliomas. J Neurooncol 121, 73–81 (2015). https://doi.org/10.1007/s11060-014-1608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-014-1608-x