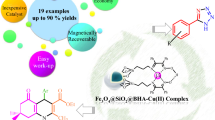
Abstract
RuIII incorporated with magnetic nanosized CMC/Fe3O4 hybrid (RuIII@CMC/Fe3O4) has readily developed by a very simple self-organized procedure of RuCl3 and Na–CMC/Fe3O4 organic/inorganic hybrid. The self-organized fresh and recovered catalyst was well characterized by ICP-AES, FTIR, XRD, TGA–DSC–DTG, SEM–EDS-mapping, TEM, and XPS techniques. The elemental maps confirmed that the RuIII species are well dispersed in a homogeneous manner on the surface of CMC/Fe3O4 magnetic hybrid nanoparticles. After full characterization, its catalytic activity was investigated in the synthesis of pyranopyrazole and polyhydroquinoline derivatives. Under optimal conditions, all reactions proceeded well and afforded the desired products in excellent yields. There was no significant effect of electron withdrawing or donating nature of substituent on aryl aldehydes in the formation of these target compounds. The salient features of the present new protocol are broad substrate scope, mild reaction conditions, good-to-excellent yields, operational simplicity, energy-efficiency, high atom-economy, easy isolation of products and no column chromatographic separation. Moreover, RuIII@CMC/Fe3O4 can be easily recovered by using a magnetic field and directly reused for several cycles without significant loss of its activity. The recovered catalyst was confirmed by TEM, XPS, XRD, and SEM–EDS analyses.
Graphical abstract
One-pot multicomponent reactions for green synthesis of pyranopyrazole and polyhydroquinoline derivatives have been performed using a self-organized RuIII@CMC/Fe3O4 nanocatalyst.















Similar content being viewed by others
References
Kuo SC, Huang LJ, Nakamura H (1984) Studies on heterocyclic compounds. 6. Synthesis and analgesic and antiinflammatory activities of 3, 4-dimethylpyrano[2,3-c]pyrazol-6-one derivatives. J Med Chem 27(4):539–544. https://doi.org/10.1021/jm00370a020
Zaki ME, Soliman HA, Hiekal OA, Rashad AE (2006) Pyrazolopyranopyrimidines as a class of anti-inflammatory agents. Z Naturforsch C 61(1–2):1–5. https://doi.org/10.1515/znc-2006-1-201
Abdelrazek FM, Metz P, Metwally NH, El-Mahrouky SF (2006) Synthesis and molluscicidal activity of new cinnoline and pyrano[2,3-c]pyrazole derivatives. Arch Pharm 339(8):456–460. https://doi.org/10.1002/ardp.200600057
Mandha SR, Siliveri S, Alla M, Bommena VR, Bommineni MR, Balasubramanian S (2012) Eco-friendly synthesis and biological evaluation of substituted pyrano[2,3-c]pyrazoles. Bioorg Med Chem Lett 22(16):5272–5278. https://doi.org/10.1016/j.bmcl.2012.06.055
Kathrotiya HG, Patel RG, Patel MP (2012) Microwave-assisted multi-component synthesis of 3ʹ-indolyl substituted pyrano[2, 3-c]pyrazoles and their antimicrobial activity. J Serb Chem Soc 77(8):983–991. https://doi.org/10.2298/JSC110805199K
Bossert F, Meyer H, Wehinger E (1981) 4-Aryldihydropyridines, a new class of highly active calcium antagonists. Angew Chem Int Ed 20(9):762–769. https://doi.org/10.1002/anie.198107621
Safak C, Simsek R (2006) Fused 1, 4-dihydropyridines as potential calcium modulatory compounds. Mini-Rev Med Chem 6(7):747–755. https://doi.org/10.2174/138955706777698606
Kumar A, Sharma S, Tripathi VD, Maurya RA, Srivastava SP, Bhatia G, Tamrakar A, Srivastava AK (2010) Design and synthesis of 2, 4-disubstituted polyhydroquinolines as prospective antihyperglycemic and lipid modulating agents. Bioorg Med Chem 18(11):4138–4148. https://doi.org/10.1016/j.bmc.2009.11.061
Al-Said MS, Ghorab MM, Al-Dosari MS, Hamed MM (2011) Synthesis and in vitro anticancer evaluation of some novel hexahydroquinoline derivatives having a benzenesulfonamide moiety. Eur J Med Chem 46(1):201–207. https://doi.org/10.1016/j.ejmech.2010.11.002
Bülbül B, Öztürk GS, Vural M, Şimşek R, Sarioğlu Y, Linden A, Ülgen M, Şafak C (2009) Condensed 1, 4-dihydropyridines with various esters and their calcium channel antagonist activities. Eur J Med Chem 44(5):2052–2058. https://doi.org/10.1016/j.ejmech.2008.10.008
Huang X, Li Z, Wang D, Li Y (2016) Bovine serum albumin: An efficient and green biocatalyst for the one-pot four-component synthesis of pyrano[2,3-c]pyrazoles. Chin J Catal 37(9):1461–1467. https://doi.org/10.1016/S1872-2067(15)61088-9
Dalal KS, Tayade YA, Wagh YB, Trivedi DR, Dalal DS, Chaudhari BL (2016) Bovine serum albumin catalyzed one-pot, three-component synthesis of dihydropyrano[2,3-c]pyrazole derivatives in aqueous ethanol. RSC Adv 6(18):14868–14879. https://doi.org/10.1039/C5RA13014J
Maleki B, Baghayeri M, Abadi SAJ, Tayebee R, Khojastehnezhad A (2016) Ultrasound promoted facile one-pot synthesis of highly substituted pyran derivatives catalyzed by silica-coated magnetic NiFe2O4 nanoparticle-supported H14[NaP5W30O110] under mild conditions. RSC Adv 6(99):96644–96661. https://doi.org/10.1039/C6RA20895A
Saha A, Payra S, Banerjee S (2015) One-pot multicomponent synthesis of highly functionalized bio-active pyrano[2,3-c] pyrazole and benzylpyrazolyl coumarin derivatives using ZrO2 nanoparticles as a reusable catalyst. Green Chem 17(5):2859–2866. https://doi.org/10.1039/C4GC02420F
Liang J, Seydimemet M, Ghalip Z, Ablajan K (2018) InCl3-catalyzed one-pot synthesis of multi-substituted pyrano[2,3-c]pyrazole-4-carboxylic acid esters under ultrasound irradiation. Mol Divers. https://doi.org/10.1007/s11030-018-9864-x
Bihani M, Bora PP, Bez G, Askari H (2013) Amberlyst A21 catalyzed chromatography-free method for multicomponent synthesis of dihydropyrano[2,3-c]pyrazoles in ethanol. ACS Sustain Chem Eng 1(4):440–447. https://doi.org/10.1021/sc300173z
Thakur A, Tripathi M, Rajesh UC, Rawat DS (2013) Ethylenediammonium diformate (EDDF) in PEG 600: an efficient ambiphilic novel catalytic system for the one-pot synthesis of 4 H-pyrans via Knoevenagel condensation. RSC Adv 3(39):18142–18148. https://doi.org/10.1039/C3RA42410C
Kanagaraj K, Pitchumani K (2010) Solvent-free multicomponent synthesis of pyranopyrazoles: per-6-amino-β-cyclodextrin as a remarkable catalyst and host. Tetrahedron Lett 51(25):3312–3316. https://doi.org/10.1016/j.tetlet.2010.04.087
Keyume A, Wang L, Yakefujiang K, Feng J (2013) Cerium ammonium nitrate (CAN)-catalyzed four-component one-pot synthesis of multi-substituted pyrano[2,3-c]pyrazoles under ultrasound irradiation. Mol Divers 17(4):693–700. https://doi.org/10.1007/s11030-013-9465-7
Zou Y, Hu Y, Liu H, Shi D (2011) Rapid and efficient ultrasound-assisted method for the combinatorial synthesis of spiro [indoline-3,4ʹ-pyrano[2,3-c]pyrazole] derivatives. ACS Comb Sci 14(1):38–43. https://doi.org/10.1021/co200128k
Elhamifar D, Badin P, Karimipoor G (2017) Alkyl-imidazolium based organosilica supported Fe/porphyrin complex: as novel, highly efficient and reusable catalyst for the unsymmetrical hantzsch reaction. J Colloid Interface Sci 499:120–127. https://doi.org/10.1016/j.jcis.2017.03.084
Maleki A, Ghassemi M, Firouzi-Haji R (2018) Green multicomponent synthesis of four different classes of six-membered N-containing and O-containing heterocycles catalyzed by an efficient chitosan-based magnetic bionanocomposite. Pure Appl Chem 90(2):387–394. https://doi.org/10.1515/pac-2017-0702
Mondal S, Patra BC, Bhaumik A (2017) One-pot synthesis of polyhydroquinoline derivatives through organic-solid-acid-catalyzed hantzsch condensation reaction. ChemCatChem 9(8):1469–1475. https://doi.org/10.1002/cctc.201601409
Zarnegar Z, Safari J, Kafroudi ZM (2015) Co3O4–CNT nanocomposites: a powerful, reusable, and stable catalyst for sonochemical synthesis of polyhydroquinolines. N J Chem 39(2):1445–1451. https://doi.org/10.1039/C4NJ01588F
Ghorbani-Choghamarani A, Azadi G (2015) Synthesis, characterization, and application of Fe3O4-SA-PPCA as a novel nanomagnetic reusable catalyst for the efficient synthesis of 2, 3-dihydroquinazolin-4(1H)-ones and polyhydroquinolines. RSC Adv 5(13):9752–9758. https://doi.org/10.1039/C4RA15315D
Yaghoubi A, Dekamin MG, Karimi B (2017) Propylsulfonic acid-anchored isocyanurate-based periodic mesoporous organosilica (PMO-ICS-PrSO3H): A highly efficient and recoverable nanoporous catalyst for the one-pot synthesis of substituted polyhydroquinolines. Catal Lett 147(10):2656–2663. https://doi.org/10.1007/s10562-017-2159-5
Rossi LM, Costa NJ, Silva FP, Wojcieszak R (2014) Magnetic nanomaterials in catalysis: advanced catalysts for magnetic separation and beyond. Green Chem 16(6):2906–2933. https://doi.org/10.1039/C4GC00164H
Karimi B, Mansouri F, Mirzaei HM (2015) Recent applications of magnetically recoverable nanocatalysts in C–C and C–X coupling reactions. ChemCatChem 7(12):1736–1789. https://doi.org/10.1002/cctc.201403057
Sharma RK, Dutta S, Sharma S, Zboril R, Varma RS, Gawande MB (2016) Fe3O4 (iron oxide)-supported nanocatalysts: synthesis, characterization, and applications in coupling reactions. Green Chem 18(11):3184–3209. https://doi.org/10.1039/C6GC00864J
Heinze T (1998) New ionic polymers by cellulose functionalization. Macromol Chem Phys 199(11):2341–2364. https://doi.org/10.1002/(SICI)1521-3935(19981101)199:11%3c2341:AID-MACP2341%3e3.0.CO;2-J
Zhang Z, Song P, Zhou J, Chen Y, Lin B, Li Y (2016) Metathesis strategy for the immobilization of copper (II) onto carboxymethylcellulose/Fe3O4 nanohybrid supports: efficient and recoverable magnetic catalyst for the CuAAC reaction. Ind Eng Chem Res 55(48):12301–12308. https://doi.org/10.1021/acs.iecr.6b03158
Du Q, Zhang W, Ma H, Zheng J, Zhou B, Li Y (2012) Immobilized palladium on surface-modified Fe3O4/SiO2 nanoparticles: as a magnetically separable and stable recyclable high-performance catalyst for Suzuki and Heck cross-coupling reactions. Tetrahedron 68(18):3577–3584. https://doi.org/10.1016/j.tet.2012.03.008
Zhang H, Deng J, Wu Y (2016) Biobased magnetic microspheres containing aldehyde groups: constructed by vanillin-derived polymethacrylate/Fe3O4 and recycled in adsorbing amine. ACS Sustain Chem Eng 5(1):658–666. https://doi.org/10.1021/acssuschemeng.6b02018
Doustkhah E, Rostamnia S, Gholipour B, Zeynizadeh B, Baghban A, Luque R (2017) Design of chitosan-dithiocarbamate magnetically separable catalytic nanocomposites for greener aqueous oxidations at room temperature. Mol Catal 434:7–15. https://doi.org/10.1016/j.mcat.2017.01.031
Eltouny NA, Ariya PA (2012) Fe3O4 nanoparticles and carboxymethyl cellulose: a green option for the removal of atmospheric benzene, toluene, ethylbenzene, and o-xylene (BTEX). Ind Eng Chem Res 51(39):12787–12795. https://doi.org/10.1021/ie3019092
Kozachuk O, Yusenko K, Noei H, Wang Y, Walleck S, Glaser T, Fischer RA (2011) Solvothermal growth of a ruthenium metal-organic framework featuring HKUST-1 structure type as thin films on oxide surfaces. Chem Commun 47(30):8509–8511. https://doi.org/10.1039/C1CC11107H
Baig RN, Nadagouda MN, Varma RS (2014) Ruthenium on chitosan: a recyclable heterogeneous catalyst for aqueous hydration of nitriles to amides. Green Chem 16(4):2122–2127. https://doi.org/10.1039/C3GC42004C
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21372099) for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, Y., Zhang, Z., Jiang, W. et al. RuIII@CMC/Fe3O4 hybrid: an efficient, magnetic, retrievable, self-organized nanocatalyst for green synthesis of pyranopyrazole and polyhydroquinoline derivatives. Mol Divers 23, 421–442 (2019). https://doi.org/10.1007/s11030-018-9887-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-018-9887-3