Abstract
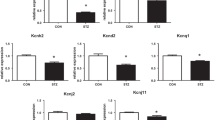
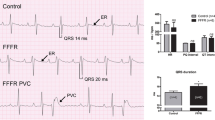
Ventricular electrical conduction has been investigated in the streptozotocin (STZ)-induced diabetic rat. Diabetes was induced with a single injection of STZ (60 mg/kg bodyweight, ip). The ECG was measured continuously, in vivo, using a biotelemetry system. Left ventricular action potentials were recorded with an extracellular suction electrode. Expression of mRNA transcripts for selected ion transport proteins was measured in left ventricle with real-time RT-PCR. At 10 weeks after STZ treatment, in vivo heart rate (HR) was reduced (267 ± 3 vs. 329 ± 5 BPM), QRS complex duration and QT interval were prolonged in diabetic rats compared to controls. In vitro spontaneous HR was reduced and paced heart action potential repolarization was prolonged in diabetic rats compared to controls. The mRNA expression for Kcnd2 (I to channel) and Kcne2 (I kr channel) was significantly reduced in diabetic rats compared to controls. Altered gene expression and, in particular, genes that encode K+ channel proteins may underlie delayed propagation of electrical activity in the ventricular myocardium of STZ-induced diabetic rat.






Similar content being viewed by others
References
Dhalla NS, Pierce GN, Innes IR, Beamish RE (1985) Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol 1:263–281
Casis O, Echevarria E (2004) Diabetic cardiomyopathy: electromechanical cellular alterations. Curr Vasc Pharmacol 2:237–248. doi:10.2174/1570161043385655
Veglio M, Chinaglia A, Cavallo-Perin P (2004) QT interval, cardiovascular risk factors and risk of death in diabetes. J Endocrinol Invest 27:175–181
Takebayashi K, Sugita R, Tayama K, Aso Y, Takemura Y, Inukai T (2003) The connection between QT dispersion and autonomic neuropathy in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 111:351–357. doi:10.1055/s-2003-42726
Rana BS, Band MM, Ogston S, Morris AD, Pringle SD, Struthers AD (2002) Relation of QT interval dispersion to the number of different cardiac abnormalities in diabetes mellitus. Am J Cardiol 90:483–487. doi:10.1016/S0002-9149(02)02518-3
Takebayashi K, Aso Y, Sugita R, Takemura Y, Inukai T (2002) Clinical usefulness of corrected QT intervals in diabetic autonomic neuropathy in patients with type 2 diabetes. Diabetes Metab 28:127–132
Veglio M, Bruno G, Borra M, Macchia G, Bargero G, D’Errico N et al (2002) Prevalence of increased QT interval duration and dispersion in type 2 diabetic patients and its relationship with coronary heart disease: a population-based cohort. J Intern Med 251:317–324. doi:10.1046/j.1365-2796.2002.00955.x
Veglio M, Chinaglia A, Cavallo PP (2000) The clinical utility of QT interval assessment in diabetes. Diabetes Nutr Metab 13:356–365
Kumhar MR, Agarwal TD, Singh VB, Kochar DK, Chadda VS (2000) Cardiac autonomic neuropathy and its correlation with QTc dispersion in type 2 diabetes. Indian Heart J 52:421–426
Veglio M, Borra M, Stevens LK, Fuller JH, Perin PC (1999) The relation between QTc interval prolongation and diabetic complications. The EURODIAB IDDM Complication Study Group. Diabetologia 42:68–75. doi:10.1007/s001250051115
Veglio M, Giunti S, Stevens LK, Fuller JH, Perin PC (2002) Prevalence of Q-T interval dispersion in type 1 diabetes and its relation with cardiac ischemia: the EURODIAB IDDM Complications Study Group. Diabetes Care 25:702–707. doi:10.2337/diacare.25.4.702
Sawicki PT, Kiwitt S, Bender R, Berger M (1998) The value of QT interval dispersion for identification of total mortality risk in non-insulin-dependent diabetes mellitus. J Intern Med 243:49–56. doi:10.1046/j.1365-2796.1998.00259.x
Naas AA, Davidson NC, Thompson C, Cummings F, Ogston SA, Jung RT et al (1998) QT and QTc dispersion are accurate predictors of cardiac death in newly diagnosed non-insulin dependent diabetes: cohort study. BMJ 316:745–746
Howarth FC, Jacobson M, Shafiullah M, Adeghate E (2008) Long-term effects of type 2 diabetes mellitus on heart rhythm in the Goto-Kakizaki rat. Exp Physiol 93:362–369. doi:10.1113/expphysiol.2007.040055
Howarth FC, Qureshi MA (2006) Effects of carbenoxolone on heart rhythm, contractility and intracellular calcium in streptozotocin-induced diabetic rat. Mol Cell Biochem 289:21–29. doi:10.1007/s11010-006-9143-5
Howarth FC, Nowotny N, Zilahi E, El Haj MA, Lei M (2007) Altered expression of gap junction connexin proteins may partly underlie heart rhythm disturbances in the streptozotocin-induced diabetic rat heart. Mol Cell Biochem 305:145–151. doi:10.1007/s11010-007-9537-z
Marionneau C, Couette B, Liu J, Li H, Mangoni ME, Nargeot J et al (2005) Specific pattern of ionic channel gene expression associated with pacemaker activity in the mouse heart. J Physiol 562:223–234. doi:10.1113/jphysiol.2004.074047
Adeghate E, al Ramadi B, Saleh AM, Vijayarasathy C, Ponery AS, Arafat K et al (2003) Increase in neuronal nitric oxide synthase content of the gastroduodenal tract of diabetic rats. Cell Mol Life Sci 60:1172–1179
Adeghate E, Ponery AS, Koves K (2000) Distribution of vasoactive intestinal polypeptide and its effect on glucagon secretion from normal and diabetic pancreatic tissue fragments in rat. Ann N Y Acad Sci 921:434–437
Howarth FC, Jacobson M, Naseer O, Adeghate E (2005) Short-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol 90:237–245. doi:10.1113/expphysiol.2004.029439
Howarth FC, Jacobson M, Shafiullah M, Adeghate E (2005) Long-term effects of streptozotocin-induced diabetes on the electrocardiogram, physical activity and body temperature in rats. Exp Physiol 90:827–835. doi:10.1113/expphysiol.2005.031252
Howarth FC, Al Sharhan R, Al Hammadi A, Qureshi MA (2007) Effects of streptozotocin-induced diabetes on action potentials in the sinoatrial node compared with other regions of the rat heart. Mol Cell Biochem 300:39–46. doi:10.1007/s11010-006-9366-5
Shigematsu S, Maruyama T, Kiyosue T, Arita M (1994) Rate-dependent prolongation of action potential duration in single ventricular myocytes obtained from hearts of rats with streptozotocin-induced chronic diabetes sustained for 30–32 weeks. Heart Vessels 9:300–306. doi:10.1007/BF01745095
Yaras N, Turan B (2005) Interpretation of relevance of sodium-calcium exchange in action potential of diabetic rat heart by mathematical model. Mol Cell Biochem 269:121–129. doi:10.1007/s11010-005-3439-8
Magyar J, Rusznak Z, Szentesi P, Szucs G, Kovacs L (1992) Action potentials and potassium currents in rat ventricular muscle during experimental diabetes. J Mol Cell Cardiol 24:841–853. doi:10.1016/0022-2828(92)91098-P
Raimondi L, De Paoli P, Mannucci E, Lonardo G, Sartiani L, Banchelli G et al (2004) Restoration of cardiomyocyte functional properties by angiotensin II receptor blockade in diabetic rats. Diabetes 53:1927–1933. doi:10.2337/diabetes.53.7.1927
Casis O, Gallego M, Iriarte M, Sanchez-Chapula JA (2000) Effects of diabetic cardiomyopathy on regional electrophysiologic characteristics of rat ventricle. Diabetologia 43:101–109. doi:10.1007/s001250050013
Shimoni Y, Firek L, Severson D, Giles W (1994) Short-term diabetes alters K+ currents in rat ventricular myocytes. Circ Res 74:620–628
Jourdon P, Feuvray D (1993) Calcium and potassium currents in ventricular myocytes isolated from diabetic rats. J Physiol 470:411–429
Pacher P, Ungvari Z, Nanasi PP, Kecskemeti V (1999) Electrophysiological changes in rat ventricular and atrial myocardium at different stages of experimental diabetes. Acta Physiol Scand 166:7–13. doi:10.1046/j.1365-201x.1999.00538.x
Lengyel C, Virag L, Biro T, Jost N, Magyar J, Biliczki P et al (2007) Diabetes mellitus attenuates the repolarization reserve in mammalian heart. Cardiovasc Res 73:512–520. doi:10.1016/j.cardiores.2006.11.010
Bracken NK, Woodall AJ, Howarth FC, Singh J (2004) Voltage-dependence of contraction in streptozotocin-induced diabetic myocytes. Mol Cell Biochem 261:235–243. doi:10.1023/B:MCBI.0000028761.61216.5e
Pandit SV, Giles WR, Demir SS (2003) A mathematical model of the electrophysiological alterations in rat ventricular myocytes in type-I diabetes. Biophys J 84:832–841. doi:10.1016/S0006-3495(03)74902-9
Shimoni Y, Ewart HS, Severson D (1998) Type I and II models of diabetes produce different modifications of K+ currents in rat heart: role of insulin. J Physiol 507:485–496. doi:10.1111/j.1469-7793.1998.485bt.x
Wang DW, Kiyosue T, Shigematsu S, Arita M (1995) Abnormalities of K+ and Ca2+ currents in ventricular myocytes from rats with chronic diabetes. Am J Physiol 269:H1288–H1296
Zeng J, Laurita KR, Rosenbaum DS, Rudy Y (1995) Two components of the delayed rectifier K+ current in ventricular myocytes of the guinea pig type. Theoretical formulation and their role in repolarization. Circ Res 77:140–152
Frank-Hansen R, Larsen LA, Andersen P, Jespersgaard C, Christiansen M (2005) Mutations in the genes KCND2 and KCND3 encoding the ion channels Kv4.2 and Kv4.3, conducting the cardiac fast transient outward current (ITO, f), are not a frequent cause of long QT syndrome. Clin Chim Acta 351:95–100. doi:10.1016/j.cccn.2004.08.017
Qin D, Huang B, Deng L, El Adawi H, Ganguly K, Sowers JR et al (2001) Downregulation of K(+) channel genes expression in type I diabetic cardiomyopathy. Biochem Biophys Res Commun 283:549–553. doi:10.1006/bbrc.2001.4825
Nishiyama A, Ishii DN, Backx PH, Pulford BE, Birks BR, Tamkun MM (2001) Altered K(+) channel gene expression in diabetic rat ventricle: isoform switching between Kv4.2 and Kv1.4. Am J Physiol 281:H1800–H1807
Guo W, Kamiya K, Toyama J (1997) Differential effects of chronic membrane depolarization on the K+ channel activities in cultured rat ventricular cells. Cardiovasc Res 33:139–146. doi:10.1016/S0008-6363(96)00191-5
Roepke TK, Kontogeorgis A, Ovanez C, Xu X, Young JB, Purtell K et al (2008) Targeted deletion of kcne2 impairs ventricular repolarization via disruption of IK, slow1 and Ito, f. FASEB J 22:3648–3660. doi:10.1096/fj.08-110171
Chun KR, Koenen M, Katus HA, Zehelein J (2004) Expression of the IKr components KCNH2 (rERG) and KCNE2 (rMiRP1) during late rat heart development. Exp Mol Med 36:367–371
Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E (2004) Pharmacology of cardiac potassium channels. Cardiovasc Res 62:9–33. doi:10.1016/j.cardiores.2003.12.026
Hattori Y, Matsuda N, Kimura J, Ishitani T, Tamada A, Gando S et al (2000) Diminished function and expression of the cardiac Na+–Ca2+ exchanger in diabetic rats: implication in Ca2+ overload. J Physiol 527:85–94. doi:10.1111/j.1469-7793.2000.00085.x
Gollob MH, Jones DL, Krahn AD, Danis L, Gong XQ, Shao Q et al (2006) Somatic mutations in the connexin 40 gene (GJA5) in atrial fibrillation. N Engl J Med 354:2677–2688. doi:10.1056/NEJMoa052800
Howarth FC, Chandler NJ, Kharche S, Tellez JO, Greener ID, Yamanushi TT et al (2008) Effects of streptozotocin-induced diabetes on connexin43 mRNA and protein expression in ventricular muscle. Mol Cell Biochem 319:105–114. doi:10.1007/s11010-008-9883-5
Nygren A, Olson ML, Chen KY, Emmett T, Kargacin G, Shimoni Y (2007) Propagation of the cardiac impulse in the diabetic rat heart: reduced conduction reserve. J Physiol 580:543–560. doi:10.1113/jphysiol.2006.123729
Lin H, Ogawa K, Imanaga I, Tribulova N (2006) Alterations of connexin 43 in the diabetic rat heart. Adv Cardiol 42:243–254. doi:10.1159/000092573
Lin H, Ogawa K, Imanaga I, Tribulova N (2006) Remodeling of connexin 43 in the diabetic rat heart. Mol Cell Biochem 290:69–78. doi:10.1007/s11010-006-9166-y
Acknowledgements
Project Grant from Faculty of Medicine & Health Sciences, UAE University
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Howarth, F.C., Jacobson, M., Qureshi, M.A. et al. Altered gene expression may underlie prolonged duration of the QT interval and ventricular action potential in streptozotocin-induced diabetic rat heart. Mol Cell Biochem 328, 57–65 (2009). https://doi.org/10.1007/s11010-009-0074-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0074-9