Abstract
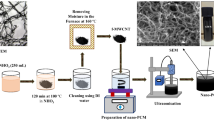
The present work deals with thermal energy storage behavior of the nano-enhanced phase change materials (NEPCMs) for building space cooling application. The NEPCMs have been prepared using Deionized (DI) water as the base phase change material (PCM) and multi-walled carbon nanotubes (MWCNTs) as nanomaterial with mass concentration of 0.25%, 0.5%, and 0.75%. For better stability of additive materials in the base PCM, sodium dodecyl-benzene sulfonate (SDBS) has been chosen as an additive element. The sub-cooling of the DI water has been completely eliminated for a mass concentration of 0.75% of MWCNT. The differential scanning calorimetry(DSC) analysis has been conducted to measure the phase transition properties of NEPCMs. The enhancements of 12.8% and 14.13% in latent heat values for charging and discharging processes, respectively, were observed for maximum mass concentration. It has also been observed that the onset temperature for charging is reduced from − 12.8 to − 9.7 °C for NEPCM with maximum concentration. The maximum thermal conductivity (k) augmentation of 23% (solid phase) and 11.2% (liquid phase) has been achieved by the NEPCM having a mass concentration of 0.75% MWCNT at − 10 °C and 15 °C, respectively. According to the study results the reductions in total charging times are 28% and 19% with the NEPCM holding a mass concentration of 0.75% MWCNT for − 8 °C and − 6 °C heat transfer fluid (HTF) temperatures, respectively. The environmental pollution remediation can be achieved by the reduction in energy input to the chiller by minimizing the total time taken for the charging the PCM.












Similar content being viewed by others
Availability of data and materials
The authors declare that all the data supporting the findings of this study are available within the article.
Abbreviations
- T:
-
Temperature (°C)
- t:
-
Time (s)
- k :
-
Thermal conductivity (W m−1 K−1)
- σ:
-
Uncertainity
- bf:
-
Base fluid
- nf:
-
Nano fluid
- logg:
-
Data logger
- inst:
-
Instrument
- DI:
-
Deionized
- PCM:
-
Phase change material
- DSC:
-
Differential scanning calorimetry
- RTD:
-
Resistance temperature detector
- SEC:
-
Specific energy consumption
- SEM:
-
Scanning electron microscope
- TEM:
-
Transmission electron microscope
- HTF:
-
Heat transfer fluid
- GNP:
-
Graphenennanoplatelets
- CTS:
-
Cool thermal storage
- LDPE:
-
Low density polyethylene
- PTDC:
-
Proportionate temperature differential controller
- MWCNT:
-
Multi wall carbon nanotubes
- NEPCM:
-
Nano-enhanced phase change material
References
Li G, Hwang Y, Radermacher R. Review of cold storage materials for air conditioning application. Int J Refrig. 2012;35:2053–77. https://doi.org/10.1016/j.ijrefrig.2012.06.003.
Li G, Hwang Y, Radermacher R. Experimental investigation on energy and exergy performance of adsorption cold storage for space cooling application. Int J Refrig. 2014;44:23–35. https://doi.org/10.1016/j.ijrefrig.2014.05.013.
Li G, Hwang Y, Radermacher R, Chun HH. Review of cold storage materials for subzero applications. Energy. 2013;51:1–17. https://doi.org/10.1016/j.energy.2012.12.002.
Cox SJ, Kim D, Cho H, Mago P. Real time optimal control of district cooling system with thermal energy storage using neural networks. Appl Energy. 2019;238:466–80. https://doi.org/10.1016/j.apenergy.2019.01.093.
Li XY, Yang L, Wang XL, Miao XY, Yao Y, Qiang QQ. Investigation on the charging process of a multi-PCM latent heat thermal energy storage unit for use in conventional air-conditioning systems. Energy. 2018;150:591–600. https://doi.org/10.1016/j.energy.2018.02.107.
Marimón MA, Arias J, Lundqvist P, Bruno JC, Coronas A. Integration of trigeneration in an indirect cascade refrigeration system in supermarkets. Energy Build. 2011;43:1427–34. https://doi.org/10.1016/j.enbuild.2011.02.003.
Krishna J, Kishore PS, Solomon AB. Heat pipe with nano enhanced-PCM for electronic cooling application. Exp Therm Fluid Sci. 2017;81:84–92. https://doi.org/10.1016/j.expthermflusci.2016.10.014.
Li G. Sensible heat thermal storage energy and exergy performance evaluations. Renew Sustain Energy Rev. 2016;53:897–923. https://doi.org/10.1016/j.rser.2015.09.0063.
Li G, Zheng X. Thermal energy storage system integration forms for a sustainable future. Renew Sustain Energy Rev. 2016;62:736–57. https://doi.org/10.1016/j.rser.2016.04.076.
Li G. Energy and exergy performance assessments for latent heat thermal energy storage systems. Renew Sustain Energy Rev. 2015;51:926–54. https://doi.org/10.1016/j.rser.2015.06.0522.
Kumaresan V, Chandrasekaran P, Nanda M, Maini AK, Velraj R. Role of PCM based nanofluids for energy efficient cool thermal storage system. Int J Refrig. 2013;36:1641–7. https://doi.org/10.1016/j.ijrefrig.2013.04.010.
Ghahremannezhad A, Xu H, Salimpour MR, Wang P, Vafai K. Thermal performance analysis of phase change materials (PCMs) embedded in gradient porous metal foams. Appl Therm Eng. 2020;179:115731. https://doi.org/10.1016/j.applthermaleng.2020.1157312.
Mesalhy O, Lafdi K, Elgafy A, Bowman K. Numerical study for enhancing the thermal conductivity of phase change material (PCM) storage using high thermal conductivity porous matrix. Energy Convers Manag. 2005;46:847–67. https://doi.org/10.1016/j.enconman.2004.06.010.
Wu S, Zhu D, Li X, Li H, Lei J. Thermal energy storage behavior of Al2O3–H2O nanofluids. ThermochimActa. 2009;483:73–7. https://doi.org/10.1016/j.tca.2008.11.006.
Leong KY, Abdul Rahman MR, Gurunathan BA. Nano-enhanced phase change materials: a review of thermo-physical properties, applications and challenges. J Energy Storage. 2019;21:18–31. https://doi.org/10.1016/j.est.2018.11.0082.
Kenisarin MM, Mahkamov K, Costa SC, Makhkamova I. Melting and solidification of PCMs inside a spherical capsule: a critical review. J Energy Storage. 2020;27:101082. https://doi.org/10.1016/j.est.2019.101082.
Li L, Yu H, Wang X, Zheng S. Thermal analysis of melting and freezing processes of phase change materials (PCMs) based on dynamic DSC test. Energy Build. 2016;130:388–96. https://doi.org/10.1016/j.enbuild.2016.08.058.
American Society for Testing and Materials, Standard test method for enthalpies of fusion and crystallization by differential scanning calorimetry. ASTM 2012;E:793–6.
Tittelein P, Gibout S, Franquet E, Johannes K, Zalewski L, Kuznik F, et al. Simulation of the thermal and energy behaviour of a composite material containing encapsulated-PCM: influence of the thermodynamical modelling. Appl Energy. 2015;140:269–74. https://doi.org/10.1016/j.apenergy.2014.11.055.
Günther E, Hiebler S, Mehling H, Redlich R. Enthalpy of phase change materials as a function of temperature: required accuracy and suitable measurement methods. Int J Thermophys. 2009;30:1257–69. https://doi.org/10.1007/s10765-009-0641-z.
Mo S, Chen Y, Jia L, Luo X. Reduction of supercooling of water by TiO2 nanoparticles as observed using differential scanning calorimeter. J Exp Nanosci. 2013;8:533–9. https://doi.org/10.1080/17458080.2011.572085.
Munyalo JM, Zhang X, Xu X. Experimental investigation on supercooling, thermal conductivity and stability of nanofluid based composite phase change material. J Energy Storage. 2018;17:47–55. https://doi.org/10.1016/j.est.2018.02.0063.
Vikram MP, Kumaresan V, Christopher S, Velraj R. Experimental studies on solidification and subcooling characteristics of water-based phase change material (PCM) in a spherical encapsulation for cool thermal energy storage applications. Int J Refrig. 2019;100:454–62. https://doi.org/10.1016/j.ijrefrig.2018.11.025.
Pabakaran R, Kumar JPN, Lal DM, Selvam C, Harish S. Constrained melting of graphene-based phase change nanocomposites inside a sphere. J Therm Anal Calorim. 2020;139:941–52. https://doi.org/10.1007/s10973-019-08458-4.
Chandrasekaran P, Cheralathan M, Velraj R. Effect of fill volume on solidification characteristics of DI (deionized) water in a spherical capsule—an experimental study. Energy. 2015;90:508–15. https://doi.org/10.1016/j.energy.2015.07.0862.
Moffat RJ. Describing the uncertainties in experimental results. Exp Therm Fluid Sci. 1988;1:3–17.
Sivashankar M, Selvam C, Manikandan S, Harish S. Performance improvement in concentrated photovoltaics using nano-enhanced phase change material with graphenenanoplatelets. Energy. 2020;208:118408. https://doi.org/10.1016/j.energy.2020.118408.
Huang L, Liu Z, Liu Y, Gou Y, Wang L. Effect of contact angle on water droplet freezing process on a cold flat surface. Exp Therm Fluid Sci. 2012;40:74–80. https://doi.org/10.1016/j.expthermflusci.2012.02.0023.
He B, Martin V, Setterwall F. Phase transition temperature ranges and storage density of paraffin wax phase change materials. Energy. 2004;29:1785–804. https://doi.org/10.1016/j.energy.2004.03.002.
Parameshwaran R, Sarı A, Jalaiah N, Karunakaran R. Applications of thermal analysis to the study of phase-change materials. Calorim: Handb. Therm. Anal; 2018.
American Society of Heating, Refrigerating and Air-conditioning Engineers. ASHRAE Handbook. Fundamentals. GA: Atlanta; 1997.
Yu W, Xie H, Bao D. Enhanced thermal conductivities of nanofluids containing graphene oxide nanosheets. Nanotechnology. 2010. https://doi.org/10.1088/0957-4484/21/5/055705.
Sati P, Shende RC, Ramaprabhu S. An experimental study on thermal conductivity enhancement of DI water-EG based ZnO(CuO)/graphene wrapped carbon nanotubes nanofluids. ThermochimActa. 2018;666:75–81. https://doi.org/10.1016/j.tca.2018.06.0084.
Mehrali M, Sadeghinezhad E, Latibari ST, Kazi SN, Mehrali M, Zubir MNBM, et al. Investigation of thermal conductivity and rheological properties of nanofluids containing graphenenanoplatelets. Nanoscale Res Lett. 2014;9:1–12.
Muthoka MJ, Xuelai Z, Xioafeng X. Study on thermophysical properties of nanofluid based composite phase change material for low temperature application. Energy Procedia. 2017;142:3313–9. https://doi.org/10.1016/j.egypro.2017.12.4633.
Khedkar RS, Shrivastava N, Sonawane SS, Wasewar KL. Experimental investigations and theoretical determination of thermal conductivity and viscosity of TiO2-ethylene glycol nanofluid. Int Commun Heat Mass Transf. 2016;73:54–61. https://doi.org/10.1016/j.icheatmasstransfer.2016.02.0043.
Assael MJ, Chen CF, Metaxa I, Wakeham WA. Thermal conductivity of suspensions of carbon nanotubes in water. Int J Thermophys. 2004;25:971–85. http://www.nanoscalereslett.com/content/9/1/15.
Xing M, Yu J, Wang R. Experimental investigation and modelling on the thermal conductivity of CNTs based nanofluids. Int J Therm Sci. 2016;104:404–11. https://doi.org/10.1016/j.ijthermalsci.2016.01.024.
Liu YD, Zhou YG, Tong MW, Zhou XS. Experimental study of thermal conductivity and phase change performance of nanofluids PCMs. MicrofluidNanofluidics. 2009;7:579–84. https://doi.org/10.1007/s10404-009-0423-8.
Wu T, Xie N, Niu J, Luo J, Gao X, Fang Y, et al. Preparation of a low-temperature nanofluid phase change material: MgCl2–H2O eutectic salt solution system with multi-walled carbon nanotubes (MWCNTs). Int J Refrig. 2020;113:136–44. https://doi.org/10.1016/j.ijrefrig.2020.02.008.
Yadav C, Sahoo RR. Thermal performance analysis of MWCNT-based capric acid PCM thermal energy storage system. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-10186-z.
Acknowledgements
The authors wish to thank the Department of Mechanical Engineering, SRM Institute of Science and Technology, Kattankulathur for the support and encouragement for carrying out this research work. The authors also like to thank the organizing committee of ICAME 2020 for the motivation and support.
Funding
The authors state that they did not receive any specific grant from the funding agencies in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
AS conducted the experiments in various surrounding conditions and consolidate the results. MC and AS interrupt the physical scenario behind the improvement of the thermo physical properties of the Phase Chance Materials (PCM)
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sathishkumar, A., Cheralathan, M. Influence of thermal transport properties of NEPCM for cool thermal energy storage system. J Therm Anal Calorim 147, 367–378 (2022). https://doi.org/10.1007/s10973-020-10339-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10339-0