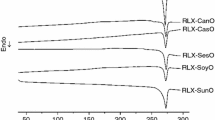
Abstract
Carvedilol (CARV) is a widely used non-selective β-blocker, which has shown low bioavailability after oral administration (20 %) due to its low water solubility and intense first-pass metabolism. Lipid-based drug delivery systems have been proposed to improve CARV oral bioavailability. An evaluation of drug–excipient compatibility is needed to clarify potential physical and chemical interactions between them and therefore guarantee a correct selection of excipients. However, to date there are no reports on the systematic evaluation of CARV–lipid excipient compatibility. Thus, the aim of this study was to evaluate the compatibility of CARV with the lipid excipients commonly used for the development of lipid-based formulations. Thermal analysis techniques (DTA and TG/DTG), Fourier transform infrared spectroscopy and isothermal stress testing (IST) were used for this purpose. The results of this study showed that 4 of the 10 lipid excipients studied were incompatible with CARV. The strongest thermal and spectroscopic modifications were observed in CARV mixtures with oleic acid, lauric acid, lauroyl polyoxylglycerides (Gelucire® 44/14) and glyceryl caprylate/caprate (Capmul® MCM). In addition, these mixtures resulted in significant decreases in drug content after aging. On the other hand, palmitic acid, stearic acid, glyceryl behenate (Compritol ATO® 188), tribeheninPEG (Emulium® 22), polyglyceryl-6-isostearate (Plurol Isostearique®) and diethylene glycol monoethyl ether (Transcutol HP®) were considered good candidates for developing self-emulsifying drug delivery systems and for preparing lipid microparticle or nanoparticle containing CARV. These findings denote the relevance of combining thermal and spectroscopic techniques with thermal stress testing for the accurate determination of drug–lipid excipient compatibility.







Similar content being viewed by others
References
Chakraborty S, Shukla D, Jain A, Mishra B, Singh S. Assessment of solubilization characteristics of different surfactants for carvedilol phosphate as a function of pH. J Colloid Interface Sci. 2009;335(2):242–9.
Dantas EM, Pimentel EB, Andreao RV, Cichoni BS, Goncalves CP, Zaniqueli Ddos A, et al. Carvedilol recovers normal blood pressure variability in rats with myocardial infarction. Auton Neurosci. 2013;177(2):231–6.
Venishetty VK, Chede R, Komuravelli R, Adepu L, Sistla R, Diwan PV. Design and evaluation of polymer coated carvedilol loaded solid lipid nanoparticles to improve the oral bioavailability: a novel strategy to avoid intraduodenal administration. Colloid Surf B. 2012;95:1–9.
Planinšek O, Kovačič B, Vrečer F. Carvedilol dissolution improvement by preparation of solid dispersions with porous silica. Int J Pharm. 2011;406(1–2):41–8.
Benet LZ. The role of BCS (biopharmaceutics classification system) and BDDCS (biopharmaceutics drug disposition classification system) in drug development. J Pharm Sci. 2013;102(1):34–42.
Singh B, Singh R, Bandyopadhyay S, Kapil R, Garg B. Optimized nanoemulsifying systems with enhanced bioavailability of carvedilol. Colloid Surf B. 2013;101:465–74.
Chakraborty S, Shukla D, Vuddanda PR, Mishra B, Singh S. Effective in vivo utilization of lipid-based nanoparticles as drug carrier for carvedilol phosphate. J Pharm Pharmacol. 2011;63(6):774–9.
Shamma RN, Basha M. Soluplus®: a novel polymeric solubilizer for optimization of Carvedilol solid dispersions: formulation design and effect of method of preparation. Powder Technol. 2013;237:406–14.
Yuvaraja K, Khanam J. Enhancement of carvedilol solubility by solid dispersion technique using cyclodextrins, water soluble polymers and hydroxyl acid. J Pharm Biomed Anal. 2014;96:10–20.
Zhang Y, Zhi Z, Li X, Gao J, Song Y. Carboxylated mesoporous carbon microparticles as new approach to improve the oral bioavailability of poorly water-soluble carvedilol. Int J Pharm. 2013;454(1):403–11.
Kalepu S, Manthina M, Padavala V. Oral lipid-based drug delivery systems—an overview. Acta Pharm Sin. 2013;3(6):361–72.
Pouton CW, Porter CJH. Formulation of lipid-based delivery systems for oral administration: materials, methods and strategies. Adv Drug Deliv Rev. 2008;60(6):625–37.
Shah MK, Madan P, Lin S. Preparation, in vitro evaluation and statistical optimization of carvedilol-loaded solid lipid nanoparticles for lymphatic absorption via oral administration. Pharm Dev Technol. 2014;19(4):475–85.
Chakraborty S, Shukla D, Mishra B, Singh S. Lipid—an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 2009;73(1):1–15.
Rao S, Tan A, Thomas N, Prestidge CA. Perspective and potential of oral lipid-based delivery to optimize pharmacological therapies against cardiovascular diseases. J Control Release. 2014;193:174–87.
Kuentz M. Lipid-based formulations for oral delivery of lipophilic drugs. Drug Discov Today Technol. 2012;9(2):e97–104.
Wei L, Sun P, Nie S, Pan W. Preparation and evaluation of SEDDS and SMEDDS containing carvedilol. Drug Dev Ind Pharm. 2005;31(8):785–94.
Salimi A, Sharif Makhmal Zadeh B, Hemati AA, Akbari Birgani S. Design and evaluation of self-emulsifying drug delivery system (SEDDS) of carvedilol to improve the oral absorption. Jundishapur J Nat Pharm Prod. 2014;9(3):e16125.
Wei L, Li J, Guo L, Nie S, Pan W, Sun P, et al. Investigations of a novel self-emulsifying osmotic pump tablet containing carvedilol. Drug Dev Ind Pharm. 2007;33(9):990–8.
Rao MRP, Munjapara GS, Khole IA. Preparation and evaluation of self-microemulsifying drug delivery system of carvedilol. Lat Am J Pharm. 2011;30(5):837–43.
Mahmoud EA, Bendas ER, Mohamed MI. Preparation and evaluation of self-nanoemulsifying tablets of carvedilol. AAPS PharmSciTech. 2009;10(1):183–92.
Mahmoud E, Bendas E, Mohamed M. Effect of formulation parameters on the preparation of superporous hydrogel self-nanoemulsifying drug delivery system (SNEDDS) of carvedilol. AAPS PharmSciTech. 2010;11(1):221–5.
Chakraborty S, Shukla D, Vuddanda PR, Mishra B, Singh S. Utilization of adsorption technique in the development of oral delivery system of lipid based nanoparticles. Colloid Surf B. 2010;81(2):563–9.
Sanjula B, Shah FM, Javed A, Alka A. Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J Drug Target. 2009;17(3):249–56.
Saindane N, Pagar K, Vavia P. Nanosuspension based in situ gelling nasal spray of carvedilol: development, in vitro and in vivo characterization. AAPS PharmSciTech. 2013;14(1):189–99.
Kuo Y-C, Chung C-Y. Solid lipid nanoparticles comprising internal Compritol 888 ATO, tripalmitin and cacao butter for encapsulating and releasing stavudine, delavirdine and saquinavir. Colloid Surf B. 2011;88(2):682–90.
Patil H, Feng X, Ye X, Majumdar S, Repka M. Continuous production of fenofibrate solid lipid nanoparticles by hot-melt extrusion technology: a systematic study based on a quality by design approach. AAPS J. 2015;17(1):194–205.
Vithani K, Cuppok Y, Mostafa S, Slipper IJ, Snowden MJ, Douroumis D. Diclofenac sodium sustained release hot melt extruded lipid matrices. Pharm Dev Technol. 2014;19(5):531–8.
Singh B, Khurana L, Bandyopadhyay S, Kapil R, Katare OO. Development of optimized self-nano-emulsifying drug delivery systems (SNEDDS) of carvedilol with enhanced bioavailability potential. Drug Deliv. 2011;18(8):599–612.
de Barros Lima Í, Lima NB, Barros DC, Oliveira T, Mendonça CS, Barbosa E, et al. Compatibility study between hydroquinone and the excipients used in semi-solid pharmaceutical forms by thermal and non-thermal techniques. J Therm Anal Calorim. 2015;120(1):719–32.
Kumar N, Bansal R, Bansal G. Evaluation of compatibility of itraconazole with excipients used to develop vesicular colloidal carriers. J Therm Anal Calorim. 2014;115(3):2415–22.
Borba P, Vecchia D, Riekes M, Pereira R, Tagliari M, Silva M, et al. Pharmaceutical approaches involving carvedilol characterization, compatibility with different excipients and kinetic studies. J Therm Anal Calorim. 2014;115(3):2507–15.
Kumar N, Goindi S, Saini B, Bansal G. Thermal characterization and compatibility studies of itraconazole and excipients for development of solid lipid nanoparticles. J Therm Anal Calorim. 2014;115(3):2375–83.
Shete H, Patravale V. Long chain lipid based tamoxifen NLC. Part I: preformulation studies, formulation development and physicochemical characterization. Int J Pharm. 2013;454(1):573–83.
Beg S, Jena SS, Patra CN, Rizwan M, Swain S, Sruti J, et al. Development of solid self-nanoemulsifying granules (SSNEGs) of ondansetron hydrochloride with enhanced bioavailability potential. Colloid Surf B. 2013;101:414–23.
Chadha R, Bhandari S. Drug–excipient compatibility screening—role of thermoanalytical and spectroscopic techniques. J Pharm Bio Anal. 2014;87:82–97.
Kasongo KW, Pardeike J, Muller RH, Walker RB. Selection and characterization of suitable lipid excipients for use in the manufacture of didanosine-loaded solid lipid nanoparticles and nanostructured lipid carriers. J Pharm Sci. 2011;100(12):5185–96.
Patil-Gadhe A, Pokharkar V. Montelukast-loaded nanostructured lipid carriers: part I oral bioavailability improvement. Eur J Pharm Biopharm. 2014;88(1):160–8.
Talvani A, Bahia M, Sá-Barreto L, Lima E, Cunha-Filho M. Carvedilol: decomposition kinetics and compatibility with pharmaceutical excipients. J Therm Anal Calorim. 2014;115(3):2501–6.
Borhade V, Pathak S, Sharma S, Patravale V. Clotrimazole nanoemulsion for malaria chemotherapy. Part I: preformulation studies, formulation design and physicochemical evaluation. Int J Pharm. 2012;431(1–2):138–48.
Hokama N, Hobara N, Kameya H, Ohshiro S, Sakanashi M. Rapid and simple micro-determination of carvedilol in rat plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1999;732(1):233–8.
Solís-Fuentes JA, Durán-de-Bazúa C. Characterization of eutectic mixtures in different natural fat blends by thermal analysis. Eur J Lipid Sci Technol. 2003;105(12):742–8.
Zhang N, Yuan Y, Yuan Y, Li T, Cao X. Lauric–palmitic–stearic acid/expanded perlite composite as form-stable phase change material: preparation and thermal properties. Energy Build. 2014;82:505–11.
Sharma A, Jain CP. Preparation and characterization of solid dispersions of carvedilol with PVP K30. Res Pharm Sci. 2010;5(1):49–56.
Stott PW, Williams AC, Barry BW. Mechanistic study into the enhanced transdermal permeation of a model β-blocker, propranolol, by fatty acids: a melting point depression effect. Int J Pharm. 2001;219(1–2):161–76.
Misic Z, Jung DS, Sydow G, Kuentz M. Understanding the interactions of oleic acid with basic drugs in solid lipids on different biopharmaceutical levels. J Excip Food Chem. 2014;5(2):113–34.
Cides LCS, Araújo AAS, Santos-Filho M, Matos JR. Thermal behaviour, compatibility study and decomposition kinetics of glimepiride under isothermal and non-isothermal conditions. J Therm Anal Calorim. 2006;84(2):441–5.
Maximiano F, Novack K, Bahia M, de Sá-Barreto L, da Cunha-Filho M. Polymorphic screen and drug–excipient compatibility studies of the antichagasic benznidazole. J Therm Anal Calorim. 2011;106(3):819–24.
Acknowledgements
The authors acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa no Estado de Goiás (FAPEG) for their financial support and Chengtai Shenyang Fine Chemical Factory and IQUEGO (Indústria Química do Estado de Goiás) for providing carvedilol.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, L.A.D., Teixeira, F.V., Serpa, R.C. et al. Evaluation of carvedilol compatibility with lipid excipients for the development of lipid-based drug delivery systems. J Therm Anal Calorim 123, 2337–2344 (2016). https://doi.org/10.1007/s10973-015-5022-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-015-5022-1