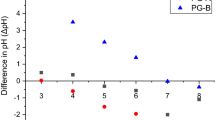
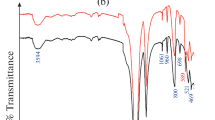
Abstract
Application study for the evaluation of sorption characteristics of sawdust as an economical sorbent material used for decontamination of radioisotopes cesium and europium from aqueous solution has been carried out in the present work. In this respect, sawdust (untreated and treated by HNO3) has been prepared from the commercial processing of wood for furniture production. Pore properties of the activated carbon such as BET surface area, pore volume, pore size distribution, and pore diameter were characterized by N2 adsorption and DFT software. Radiotracer method onto sawdust from aqueous solutions was studied in a batch technique with respect to pH, contact time, temperature. The kinetics of adsorption of Eu3+ and Cs+ have been discussed using five kinetic models namely, pseudo-first-order model, pseudo-second-order model, Elovich equation, intraparticle diffusion model, and modified Freundlich equation that have been tested in order to analysis the experimental data. Kinetic parameters and correlation coefficients were determined. It was shown that the second-order kinetic equation could describe the sorption kinetics for two metal ions. The metal uptake process was found to be controlled by intraparticle diffusion. Thermodynamic parameters, such as ΔH, ΔG and ΔS, have been calculated by using the thermodynamic equilibrium coefficient obtained at different temperatures. The obtained results indicated that endothermic nature of sorption process for both 152+154Eu and 134Cs onto sawdust.









Similar content being viewed by others
References
David Liu HF, Liptak GB (2000) Hazardous waste and solid waste. Lewis Publishers, USA
International Atomic Energy Agency (IAEA) (1992) Use of inorganic sorbents for treatment of liquid radioactive waste and backfill of underground repositories. IAEA-TECDOC-675, Vienna
International Atomic Energy Agency (IAEA) (2002) Technical Reports Series, No. 408, ‘‘Application of ion exchange processes for the treatment of radioactive waste and management of spent ion exchangers’’, IAEA, Vienna, Austria
International Atomic Energy Agency (IAEA) (2003) Combined methods for liquid radioactive waste treatment. IAEA-TECDOC-1336, Vienna, Austria
Hameed BH, Ahmad AL, Latiff KNA (2007) Dyes Pigment 75:143–149
Shukla A, Zhang Y, Dubey P, Margrave JL, Shukla SS (2002) J Hazard Mater B95:137–152
Srinivasakannan C, Abu MZ (2004) Bakar, Biomass Bioenergy 27:89–96
Zacar MO, Sengil IA (2005) Bioresour Technol 96:791–795
Deshkar AM, Bokade SS, Dara SS (1990) Water Res 24:1011–1016
Seki K, Saito N, Aoyama M (1997) Wood Sci Technol 31:441–447
Šćiban M, Klašnja M, Škrbić B (2006) J Hazard Mater B136:266–271
Gupta S, Babu BV (2009) Chem Eng J 150:352–365
Karthikeyan T, Rajgopal S, Miranda LR (2005) J Hazard Mater B124:192–199
Sreejalekshmi KG, Krishnan KA, Anirudhan TS (2009) J Hazard Mater 161:1506–1513
Ahmada A, Rafatullah M, Sulaiman O, Ibrahim MH, Chii YY, Siddique BM (2009) Desalination 247:636–646
Vijayaraghavan K, Won SW, Yun YS (2009) J Hazard Mater 167:790–796
Khattri SD, Singh MK (2009) J Hazard Mater 167:1089–1094
Sing KW, Everet DH, Haul RAW, Moscou L, Pierotti RA, Rouquero J, Siemieniewasa T (1985) Pure Appl Chem 57:603–619
Ryu Z, Zheng J, Wang M, Zhang B (1999) Carbon 37:1257–1264
Seaton NA, Walton JPRB, Quirke N (1989) Carbon 27:853–861
Puziy AM, Poddubnaya OI, Martnez-Alonso A, Suarez-Garcia F, Tascon JMD (2002) Carbon 40:1493–1505
Pradhan BK, Sandles NK (1999) Carbon 37:1323–1332
El-Sheikh AH, Newman AP, Al-Daffaee HK, Phull S, Cresswell N, Anal J (2004) Appl Pyrolysis 71:151–164
Menedez JA, Menendez EM, Iglesias MJ, Garcia A, Pis JJ (1999) Carbon 37:1115–1121
Mehandjiev DR, Nickolov RN, Ioncheva RB (1997) Fuel 76(5):381–384
Shim JW, Park SJ, Ryu SK (2001) Carbon 39:1635–1642
Batzias FA, Sidiras DK (2004) J Hazard Mater B114:167–174
Shuklaa SS, Yua LJ, Dorrisa KL, Shukla A (2005) J Hazard Mater B121:243–246
Abdel-Galil EA (2006) Chemical studies for sorption of some radionuclides on silico (IV) titanate as cation exchanger, M.Sc. thesis, Chemistry Department, Faculty of Science, Zagazig University
Shehata FA, Attallah MF, Borai EH, Hilal MA, Abo-Aly MM (2010) Appl Radiat Isot 68:239–249
Barrett PEP, Joyner LG, Halenda PP (1951) J Am Chem Soc 73:373–380
Ho YS, McKay G (1998) Chem Eng J 70:115–124
Cheung CW, Porter JF, Mckay G (2000) Sep Purif Technol 19:55–64
Weber WJ Jr, Morris JC (1963) J Saint Eng Div Am Soc Civil Eng 89:31–60
Kuo S, Lotse EG (1973) Soil Sci Soc Am J 116:400–406
Teng H, Hsieh C (1999) Ind Eng Chem Res 38:292–297
Argun ME, Dursun S, Ozdemir C, Karatas M (2007) J Hazard Mater 141:77–85
Ofomaja AE (2008) Chem Eng J 143:85–95
Kaczala F, Marques M, Hogland W (2009) Bioresour Technol 100:235–243
Özacar M, Sengil IA (2005) Process Biochem 40:565–572
Rudzinski W, Panczyk T (2002) Adsorption 8:23–34
Gökmen V, Serpen A (2002) J Food Eng 53:221–227
Aharoni C, Tompkins FC (1970) Kinetics of adsorption and desorption and the Elovich equation. In: Eley DD, Pines H, Weisz PB (eds) Advances in catalysis and related subjects, vol 21. Academic Press, New York, pp 1–49
Ru-Ling T, Feng-Chin W, Ruey-Shin J (2003) Carbon 41:487–495
Ayyappan R, Carmalin Sophia A, Swaminathan K, Sandhya S (2005) Process Biochem 40:1293–1299
Özacar M, Sengil IA (2004) Biochem Eng J 21:39–45
Arslano¢glu FN, Kar F, Arslan N (2005) J Food Eng 68:409–417
Saleem M, Afzal M, Qadeer R, Hanif J (1992) Sep Sci Technol 27(2):239–253
Gupta VK, Mohan D, Sharma S (1998) Sep Sci Technol 33(9):1331–1343
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hassan, H.S., Attallah, M.F. & Yakout, S.M. Sorption characteristics of an economical sorbent material used for removal radioisotopes of cesium and europium. J Radioanal Nucl Chem 286, 17–26 (2010). https://doi.org/10.1007/s10967-010-0654-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0654-x