Abstract
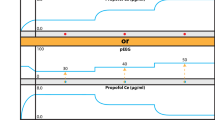
During emergence from anesthesia patients regain their muscle tone (EMG). In a typical population of surgical patients the actual volatile gas anesthetic concentrations in the brain (CeMAC) at which EMG activation occurs remains unknown, as is whether EMG activation at higher CeMACs is correlated with subsequent severe pain, or with cortical activation. Electroencephalographic (EEG) and EMG activity was recorded from the forehead of 273 patients emerging from general anesthesia following surgery. We determined CeMAC at time of EMG activation and at return of consciousness. Pain was assessed immediately after return of consciousness using an 11 point numerical rating scale. The onset of EMG activation during emergence was associated with neither discernible muscle movement nor with the presence of exogenous stimulation in half the patients. EMG activation could be modelled as two distinct processes; termed high- and low-CeMAC (occurring higher or lower than 0.07 CeMAC). Low-CeMAC activation was typically associated with simultaneous EMG activation and consciousness, and the presence of a laryngeal mask. In contrast, high-CeMAC EMG activation occurred independently of return of consciousness, and was not associated with severe post-operative pain, but was more common in the presence of an endotracheal tube. Patients emerging from general anesthesia with an endotracheal tube in place are more likely to have an EMG activation at higher CeMAC concentrations. These activations are not associated with subsequent high-pain, nor with cortical arousal, as evidenced by continuing delta waves in the EEG. Conversely, patients emerging from general anesthesia with a laryngeal mask demonstrate marked neural inertia—EMG activation occurs at a low CeMAC, and is closely temporally associated with return of consciousness.






Similar content being viewed by others
References
Aranake A, Mashour GA, Avidan MS. Minimum alveolar concentration: ongoing relevance and clinical utility. Anaesthesia. 2013;68:512–22.
Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia–brainstem pathways in the control of motor behaviors. Neurosci Res. 2004;50:137–51.
Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, et al. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–12.
Dutton RC, Smith WD, Bennett HL, Archer S, Smith NT. Craniofacial electromyogram activation response: another indicator of anesthetic depth. J Clin Monit Comput. 1998;14:5–17.
Edmonds HL, Couture LJ, Stolzy SL, Paloheimo M. Quantitative surface electromyography in anesthesia and critical care. Int J Clin Monit Comput. 1986;3:135–45.
Paloheimo M. Quantitative surface electromyography (qEMG): applications in anaesthesiology and critical care. Acta Anaesthesiol Scand Suppl. 1989;93:1–83.
Edmonds HL, Couture LJ, Paloheimo MP, Rigor BM. Objective assessment of opioid action by facial muscle surface electromyography (SEMG). Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:727–38.
Bonhomme V, Hans P. Muscle relaxation and depth of anaesthesia: where is the missing link? Br J Anaesth. 2007;99:456–60.
Aho AJ, Lyytikainen L-P, Yli-Hankala A, Kamata K, Jantti V. Explaining Entropy responses after a noxious stimulus, with or without neuromuscular blocking agents, by means of the raw electroencephalographic and electromyographic characteristics. Br J Anaesth. 2011;106:69–76.
Chander D, García PS, MacColl JN, Illing S, Sleigh JW. Electroencephalographic variation during end maintenance and emergence from surgical anesthesia. PLoS one. 2014;9:106291.
Hight DF, Dadok VM, Szeri AJ, Garci-a PS, Voss L, Sleigh JW. Emergence from general anesthesia and the sleep-manifold. Front Syst Neurosci. 2014;8. http://journal.frontiersin.org/Journal/10.3389/fnsys.2014.00146/full.
Kennedy R, McKellow M, French R, Sleigh J. Sevoflurane end-tidal to effect-site equilibration in women determined by response to Laryngeal mask airway insertion. Anesth Analg. 2013;117:786–91.
McKay IDH, Voss LJ, Sleigh JW, Barnard JP, Johannsen EK. Pharmacokinetic-pharmacodynamic modeling the hypnotic effect of sevoflurane using the spectral entropy of the electroencephalogram. Anesth Analg. 2006;102:91–7.
Plaud B, Proost JH, Wierda J, Barre J, Debaene B, Meistelman C. Pharmacokinetics and pharmacodynamics of rocuronium at the vocal cords and the adductor pollicis in humans. Clin Pharmacol Ther. 1995;58:185–91.
Yassen A. Pharmacokinetic-pharmacodynamic modeling of the antinociceptive effect of buprenorphine and fentanyl in rats: role of receptor equilibration kinetics. J Pharmacol Exp Ther. 2005;313:1136–49.
Mazoit JX, Butscher K, Samii K. Morphine in postoperative patients: pharmacokinetics and pharmacodynamics of metabolites. Anesth Analg. 2007;105:70–8.
Naguib M, Samarkandi AH, Bakhamees HS, Magboul MA, el-Bakry AK. Comparative potency of steroidal neuromuscular blocking drugs and isobolographic analysis of the interaction between rocuronium and other aminosteroids. Br J Anaesth. 1995;75:37–42.
Law C, Sleigh J, Barnard J, MacColl J. The association between intraoperative electroencephalogram-based measures and pain severity in the post-anaesthesia care unit. Anaesth Intensive Care. 2011;39:875–80.
Mitra P, Bokil H. Observed brain dynamics. Oxford: Oxford University Press; 2008.
Loader C. LOCFIT: an introduction. Stat Comput Graph Newsl. 1997;8:11–7.
Hosmer DW Jr, Lemeshow S. Applied logistic regression. New York: Wiley; 2004.
Friedman EB, Sun Y, Moore JT, Hung H-T, Meng QC, Perera P, et al. A conserved behavioral state barrier impedes transitions between anesthetic-induced unconsciousness and wakefulness: evidence for neural inertia. PLoS one. 2010;5(7):e11903.
Kungys G, Kim J, Jinks SL, Atherley RJ, Antognini JF. Propofol produces immobility via action in the ventral horn of the spinal cord by a GABAergic mechanism. Anesth Analg. 2009;108:1531–7.
Jinks SL, Bravo M, Hayes SG. Volatile anesthetic effects on midbrain-elicited locomotion suggest that the locomotor network in the ventral spinal cord is the primary site for immobility. Anesthesiology. 2008;108:1016–24.
Mazzone SB, McGovern AE, Farrell MJ. Endogenous central suppressive mechanisms regulating cough as potential targets for novel antitussive therapies. Curr Opin Pharmacol. 2015;22:1–8.
Taylor-Clark TE. Peripheral neural circuitry in cough. Curr Opin Pharmacol. 2015;22:9–17.
Brown EN, Lydic R, Schiff ND. General anesthesia, sleep, and coma. N Engl J Med. 2010;363:2638–50.
Jospin M, Caminal P, Jensen EW, Litvan H, Vallverdu M, Struys MMRF, et al. Detrended fluctuation analysis of EEG as a measure of depth of anesthesia. IEEE Trans Biomed Eng. 2007;54:840–6.
Aho AJ, Kamata K, Jäntti V, Kulkas A, Hagihira S, Huhtala H, et al. Comparison of bispectral index and entropy values with electroencephalogram during surgical anaesthesia with sevoflurane. Br J Anaesth. 2015;115:258–66.
Schuller PJ, Newell S, Strickland PA, Barry JJ. Response of bispectral index to neuromuscular block in awake volunteers. Br J Anaesth. 2015;115:i95–i103.
Acknowledgments
This project has been funded with funds from the James S. McDonnell Foundation under Grant Award No. 220020346. The results were independently derived and do not reflect any endorsement on the part of the James S. McDonnell Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Northern Y Regional Ethics Committee of New Zealand (NTY/11/EXP/077) and the New Zealand Health and Disability Ethics Committee (12/CEN/56) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study. This article does not contain any studies with animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Hight, D.F., Voss, L.J., García, P.S. et al. Electromyographic activation reveals cortical and sub-cortical dissociation during emergence from general anesthesia. J Clin Monit Comput 31, 813–823 (2017). https://doi.org/10.1007/s10877-016-9911-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-016-9911-z