Abstract
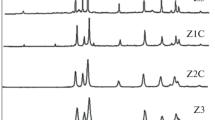
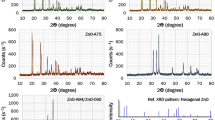
The reaction of aliphatic alcohols such as methanol, ethanol and t-butanol with zinc powder at 330 °C under solvothermal conditions produces ZnO nanoparticles. The reaction involves the cleavage of the C-O bond of the alcohols, which occurs readily on the Zn metal surface. Addition of ethylenediamine to the reaction mixture yields nanorods, the amine acting as a shape-controlling agent.







Similar content being viewed by others
References
Z. L. Wang (2004) Mater. Today 7:26
C. N. R. Rao and A. Govindaraj, in H. Kroto, P. O’Brien and H. Craighead (eds.), Nanotubes and Nanowires, The RSC Nanoscience & Nanotechnology series (Royal Society of Chemistry, London, 2005)
H. Usui et al (2005) J. Phys. Chem. B 109:120
E. M. Wong P. C. Searson (1999) Appl. Phys. Lett. 74:2939
M. Ghosh et al (2004) J. Nanosci. Nanotechnol. 4:136
U. Koch et al (1985) Chem. Phys. Lett. 122:507
M. D. Mustafa et al (2006) J. Mater. Chem. 16:2940
J. Buha et al (2007) Crystal Growth & Design 7:113
M. Shim P. Guyot-Sionnest (2001) J. Am. Chem. Soc. 123:11651
M. Monge et al (2003) Angew.Chem. Int. Ed. 42:5321
L. Spanhel M. A. Anderson (1991) J. Am. Chem. Soc. 113:2826
M.S. Tokumoto et al (2003) J. Phys. Chem. B 107:568
B. J. Joo et al (2005) Adv. Mater. 17:1873
Z. R. Qiu et al (2004) Appl. Phys. Lett. 84:2739
B. P. Zhang et al (2004) Appl. Phys. Lett. 84:586
S. F. Yu et al (2004) Appl. Phys. Lett. 84:3241
Q. X. Zhao et al (2003) Appl. Phys. Lett. 83:165
C. J. Lee et al (2002) Appl. Phys. Lett. 81:3648
J. J. Wu S. C. Liu (2002) Adv. Mater. 14:215
X. Liu et al (2004) J. Appl. Phys. 95:3141
Y. Zhang et al (2003) Appl. Phys. Lett. 83:4631
Y. C. Kong et al (2001) Appl. Phys. Lett. 78:407
S. C. Lyu et al (2003) Chem. Mater. 15:3294
W.I. Park et al (2002) Appl. Phys. Lett. 80:4232
Y. Li et al. (2000) Appl. Phys. Lett. 76:2011
C. Liu et al (2003) Adv. Mater. 15:838
H. Yu et al (2005) J. Am. Chem. Soc. 127:2378
B. Cheng et al (2006) Inorg. Chem. 45:1208
C. Wang et al (2005) Mater. Lett. 59:2867
B. Liu H. C. Zeng (2003) J. Am. Chem. Soc. 125:4430
A. Dev et al (2006) Nanotechnology 17:1533
K. R. Harikumar et al (1999) J. Phys. Chem. B 103:2445
K. R. Harikumar and C.N.R. Rao (1999). Chem. Commun. 341
N. S. Pesika et al (2003) J. Phys. Chem. B 107:10412
X. Q. Meng et al (2005) Solid State Commun. 135:179
K. Vanheusden et al (1996) J. Appl. Phys. 79:7983
Acknowledgements
One of the authors (LSP) acknowledges CSIR, New Delhi, for a junior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Dieter Fenske
Rights and permissions
About this article
Cite this article
Panchakarla, L.S., Govindaraj, A. & Rao, C.N.R. Formation of ZnO Nanoparticles by the Reaction of Zinc Metal with Aliphatic Alcohols. J Clust Sci 18, 660–670 (2007). https://doi.org/10.1007/s10876-007-0129-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-007-0129-6