Abstract
Purpose
Standard therapy for lupus nephritis is based on non-specific immunosuppression. We aimed to identify specific alterations in T cell and cytokine homeostasis and possible associations with disease activity in children with lupus nephritis (LN).
Methods
The phenotype of circulating T cells from children with LN and healthy controls (HC) was analyzed by flow cytometry. Intracellular expression of IL-17 and INF-γ was assessed after stimulation with anti-CD3 and anti-CD28. Serum concentrations of IP10, CCL2, TGF-β, IL-17, and IL-23 were measured by ELISA. Disease activity was determined using the Systemic Lupus Erythematosus Disease Activity Index 2000 update (SLEDAI-2K).
Results
Children with active LN displayed increased frequencies of effector memory CD4+CD45RO+CCR7− and terminal differentiated CD4+CD45RA+CCR7− T cells and reduced naive CD4+CD45RA+CCR7+ T cells compared to those with inactive LN or HC. Circulating CD4+CXCR3+ and CD4+CCR2+ T cells correlated inversely with the renal SLEDAI-2K, whereas IP10 and CCL2 showed a positive correlation. Reduced CD4+Foxp3+ T cells and serum TFG-β levels in active LN were associated with high serum IL-17 and IL-23 levels and correlated inversely with the renal SLEDAI-2K (r = −0.5855, p = 0.0013 and r = −0.6246, p = 0.0005, respectively), whereas IL-17 and IL-23 correlated positively (r = 0.5516, p = 0.0029 and r = 0.6116, p = 0.0007, respectively). Expansion of Th17 and Th1/Th17 cells in children with LN was significantly greater than in HC (p = 0.0304 and p = 0.0067, respectively).
Conclusion
Children with active LN display high levels of pro-inflammatory cytokines associated with an increase in effector T cells and reduction of regulatory T cells. Therapeutic regulation of the aberrant cytokine profile might specifically interrupt pathogenic mechanisms.
Similar content being viewed by others
Introduction
T cells play a central role in the pathogenesis of systemic lupus erythematosus (SLE) and lupus nephritis (LN) [1]. Lymphocyte infiltrates and interstitial inflammation correlates with the extent of renal injury and the progression to renal insufficiency [2].
Various T cell abnormalities have been described in adult patients with SLE, including a reduced threshold of activation [3], increased frequencies of double negative (DN) T cells [4, 5] and Th17 cells [6], an enhanced expression of co-stimulatory molecules [6] and of chemokine receptors on renal-infiltrating T cells [7] along with high intra-renal concentrations of diverse chemokines [7]. This illustrates that multiple distinct processes are involved in the initiation and progression of LN. Regulatory T cells are essential for the maintenance of self-tolerance and immune homeostasis [8], and deficiency in immunoregulation has a critical impact on the development of autoreactivity [9]. However, the findings regarding the quantity and function of regulatory T cells in adult SLE patients are contradictory [10]. Due to this enormous complexity, the underlying mechanisms of LN remain elusive.
Children with LN have a more severe disease at onset and a more aggressive clinical course and incur permanent damage at a more rapid rate than patients with adult-onset SLE [11]. The risk of progression to end-stage renal disease in childhood LN is up to 50% [12]. Thus, compared to adults, children usually receive a more intensive drug therapy [11]. However, current standard treatment regimens are based on non-specific immunosuppression and are associated with considerable side effects and toxicity in especially vulnerable phases of growth and development [11].
Nonetheless, little is known regarding the disturbances of T cell homeostasis in childhood LN. The investigation into factors involved in the immunological imbalance of childhood LN will help to identify molecules that can be targeted to achieve disease suppression without interfering with the function of the protective immune system. To address these important questions, we explored in children with LN and control subjects the phenotype of circulating T cells, the cytokine milieu, and in vitro differentiation of Th17, Th1/Th17, and Th1 cells and assessed correlations with LN activity.
Patients and Methods
Study Population
Twenty-three patients with definite lupus nephritis, 12 patients with frequently relapsing nephrotic syndrome (minimal change glomerulonephritis or focal segmental glomerulosclerosis), and 20 age-matched healthy controls were enrolled in the study and details are given in Table I. Patients with LN (18 females and five males) were from the Department of Pediatrics in Innsbruck (A), Heidelberg (G), Rostock (G), Muenchen (G), Erlangen (G), Tuebingen (G), and Memmingen (G). The patients all have SLE diagnosed according to the American College of Rheumatology revised classification criteria for definite SLE with disease onset prior to 16 years of age [13, 14]. Histology of renal biopsies was classified according to the International Society of Nephrology and the Renal Pathology Society [15].
Patients with frequently relapsing minimal change nephrotic syndrome or focal segmental glomerulosclerosis (two females and 10 males, 10 during the non-nephrotic phase, two with persistent proteinuria) all under immunosuppressive therapy were from the Department of Pediatrics in Innsbruck (A). These patients were included to assess the effects of immunosuppression. Age-matched healthy children (five females and 15 males) undergoing examination and blood tests for sport eligibility evaluation or elective surgery for hernia inguinalis, umbilicalis, or phimosis at the Department of Surgery Innsbruck Medical University served as healthy controls.
Blood samples for the study were collected from all 23 patients with LN and controls on the occasion of routine blood testing at first diagnosis (n = 3 patients with LN), disease flare (n = 12 patients with LN), remission (n = 12 patients with LN), surgery, or examination for sport eligibility evaluation. From 4 out of 23 patients with LN, blood samples were obtained twice during active as well as inactive LN.
Written informed consent was obtained from all patients and parents prior to inclusion. The study has been approved by the local ethical committees and has been performed in accordance with the 1964 Declaration of Helsinki and its later amendments.
Assessment of Disease Activity and Renal Damage
Disease activity was assessed at the time of sampling by the Systemic Lupus Erythematosus Disease Activity Index 2000 update (SLEDAI-2K) [16, 17]. Active SLE disease was defined as a SLEDAI-2K score of ≥4 and inactive SLE disease as a SLEDAI-2K score of <4. Lupus nephritis activity was defined according to the scores in the renal domain of the SLEDAI-2K (active renal disease: renal SLEDAI-2K score ≥4 with hematuria (>5 red blood cells/high power field, stones or infection were excluded) or/and pyuria (>5 white blood cells/ high power field, infection was excluded) or/and urinary casts (heme-granular or red blood cell casts) or/and proteinuria >0.5 g/24 h; inactive renal disease: renal domain score of SLEDAI-2K = 0) [16]. Renal damage was measured using the renal domain of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology SLE Damage Index (renal SDI score) [16].
Measurement of Cytokine Concentrations
IP-10, CCL2, IL-17, TGF-beta 1 (TGF-β), and IL-23 concentrations in serum samples were measured by using commercially available ELISA kits (Human CXCL10/IP-10 Quantikine ELISA Kit, Human CCL2/MCP-1 Quantikine ELISA Kit, Human IL-17 Immunoassay, Human TGF-beta1 Immunoassay, Human IL-23 Immunoassay, Quantikine®, R&D Systems, Inc., Minneapolis, MN, USA), following the manufacturer’s instructions.
Immune Phenotype Analysis
Multicolor flow cytometry studies were performed on EDTA blood samples (Beckman Coulter FC500, CXP-Acquisition software). All samples were processed within the first 24 h after collection. Numbers of leukocytes and lymphocytes were calculated using the Sysmex XE-2100 hematology analyzer. Samples were then prepared using a direct immunofluorescence stain-and-then-lyse technique. After staining the surface antigens, cells were permeabilized to detect intracellular markers using IntraPrep™ Permeabilization Reagent (Beckman Coulter, Inc., Brea, CA, USA) according to the manufacturer’s instructions. The following monoclonal antibodies were used: CD3 FITC/CD16/56 PE, CD3 FITC/CD25 PE, CD3 ECD, CD4 ECD, CD4 PC5, CD8 PC5, CD8 PC7, HLA-DR FITC, HLA-DR ECD, CD14 PC5, CD19 PC5, CD19 FITC, CD45 PC7, CD28 FITC, CD45RO FITC, CD134 PE, CD69 PE, CD80 PE, CD45RA ECD, CD20 ECD, CD25 ECD (Beckman Coulter, Inc.), CCR2 PE, CCR7 PE (R&D Systems, Inc.), CXCR3 FITC (BD, Franklin Lakes, NJ, USA), Foxp3 FITC (eBioscience, San Diego, CA, USA), and appropriate isotype control antibodies. For data analysis, CXP-Analysis software program was used.
Preparation of PBMC and In Vitro Stimulation
In order to evaluate Th17, Th1/Th17, and Th1 cells, human peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Paque PLUS (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) density gradient centrifugation of EDTA blood samples from 12 patients with LN (active LN, n = 5; inactive LN, n = 7) and 12 healthy controls. PBMC were cultured in 96-well round-bottom plates (BD Biosciences) in complete RPMI-1640 medium (2 mM l-glutamine, 10% heat-inactivated FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin) and rested for 3 days. Cells were then stimulated in complete RMPI-1640 medium with anti-Human CD3 functional grade purified (OKT3) (eBioscience, 1 μg/ml) and anti-Human CD28 functional grade purified (CD28.6) (eBioscience, 1 μg/ml) for 7 days and restimulated with Leukocyte Activation Cocktail with GolgiPlug™ (BD Pharmingen, NJ, USA) during the final 4 h. Cells were fixed and permeabilized with Foxp3 Staining Buffer Set (eBioscience), then stained with CD3 ECD (Beckman Coulter, Inc.), IL-17A PE 64DEC17, (eBioscience) and INF-γ PE-Cy7 (eBioscience) or appropriate isotype control antibodies, washed, and analyzed by flow cytometry (Beckman Coulter).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism 5.0 (San Diego, CA, USA). Data are presented as mean ± SEM, unless stated otherwise. Differences between groups were calculated using the nonparametric Mann–Whitney U test or Kruskal–Wallis test, and correlation analyses were performed using the Spearman’s test. For all tests, p values of <0.05 were considered significant.
Results
Study Population
The characteristics of the study population and the given immunosuppressive therapy are shown in Table I. Extra-renal descriptors of the SLEDAI score in all patients included low complement (n = 13), increased DNA binding (n = 7), new rash (n = 4), leukopenia (n = 2), thrombocytopenia (n = 1), and arthritis (n = 1). Details on a patient-by-patient basis in patients with active LN are given in Online Resource 1. At the time of sampling, three patients with active LN had no medication because of first diagnosis and the other patients were under immunosuppressive treatment as specified in Table I. Six patients with active LN and ten patients with inactive LN had received intravenous pulse cyclophosphamide (cumulative dose 3,000 mg/m2 (1,500–3,500 mg/m2) and 3,250 mg/m2 (1,500–6,000 mg/m2), respectively), a median of 42 months (4–72) and 28 months (3–60), respectively, prior to enrollment into the study (Table I). The median duration between diagnosis and treatment of active LN and blood withdrawal was 10 days (range 0 to 335 days) and for inactive LN 396 days (range 31 to 1,006 days). Median duration of disease since first diagnosis in patients with active LN was 7 months (range 0–84 months), in those with inactive LN 34 months (range 4–90 months), and in patients with nephrotic syndrome 44 months (range 5–120 months).
Reduced Numbers of Circulating Leukocytes and T Cells in Childhood LN
The median number of peripheral blood leukocytes, CD4+, and CD8+ T cells was decreased in children with active LN 4,660/μl (range 1,076–8,460), 475/μl (range 191–1,426), and 379/μl (range 122–578), respectively, compared to children with inactive LN 6,450/μl (range 3,430–11,440), p = 0.1261; 590/μl (range 93–1,035), p = 0.6430; and 499/μl (range 210–2,316), p = 0.5781, respectively, or healthy controls (HC) 7,080/μl (range 4,380–9,260), p = 0.0368; 1,067/μl (range 725–1,781), p < 0.0001; and 708/μl (range 387–1,423), p < 0.0001, respectively.
Increased Frequency of Activated CD4+ and CD8+ T Cells in Childhood LN
As shown in Table II, children with LN displayed a significantly higher percentage of CD69 and HLA-DR expressing CD4+ T cells and of HLA-DR expressing CD8+ T cells compared with children with NS or HC. The mean frequency of CD4+CD134+ T cells within the CD4+ T cell population was significantly higher in children with LN than children with NS or HC. Though absolute CD4+ and CD8+ T cell counts were significantly reduced in LN, mean absolute numbers of activated T cells did not significantly differ between patients with LN, NS, or HC, except for CD4+CD134+ cells (Table II). No significant difference was noted between active and inactive LN in the mean frequencies of CD69, HLA-DR, or CD134 expressing CD4+ or CD8+ T cells. Overall expression of CD134 on CD8+ T cells was very low and not significantly different between the groups (0.15 ± 0.03% for LN, 0.15 ± 0.07% for NS, 0.05 ± 0.03% for HC). There was no significant difference in the percentage of CD4+CD25+ T cells from LN patients (5.9 ± 0.9%) compared with HC (4.1 ± 0.8%, p = 0.326). CD80 expression was not detectable on either CD4+ or CD8+ T cells in none of the patients or controls analyzed in this study.
Enhanced CD4+ and CD8+ T Cell Differentiation in Children with LN
The frequencies of naive CD45RA+CCR7+ CD4+ and CD8+ T cells in patients with LN were significantly less than in HC (Table III) whereas those of CD4+ and CD8+ effector memory T cells (EM, CD45RO+CCR7−) were significantly greater in LN than in HC (Table III and Online Resource 2).
In active LN compared to inactive LN, the frequencies of naive CD4+ T cells were significantly reduced, while EM CD4+ T cells and terminal differentiated CD4+ T cells (TD, CD45RA+CCR7−) were enhanced (Table III). No significant difference however was observed in the frequencies of naive CD8+, EM CD8+, and TD CD8+ T cells between active and inactive LN (Table III).
Expansion of Activated Double Negative T Cells in Childhood LN
Figure 1a and Online Resource 3 shows that the mean frequency of circulating double negative T cells (DN T cells, CD3+CD4−CD8−) within in the CD3+CD16/56− T cell population was significantly lower in active compared with inactive LN (p = 0.0454) and HC (p = 0.0118). DNT cells were also absolutely reduced in active LN (43 ± 7/μl) compared with inactive LN (85 ± 16/μl, p = 0.023), NS (174 ± 30/μl, p = 0.0002), or HC (130 ± 13/μl, p < 0.0001). The percentage of activated CD69 expressing cells within the DN T cell subset was significantly higher in active LN compared with NS (p = 0.0008) or HC (p = 0.0015) (Fig. 1b). No significant difference however was noted between children with active and inactive LN (p = 0.272, Fig. 1b). Likewise, though the mean frequency of HLA-DR expressing DN T cells was similar in patients with active and inactive LN (p = 0.1807), it was significantly higher in active LN patients compared with NS (p = 0.005) or HC (p = 0.004) (Fig. 1c). Though total CD3+ T cell numbers were significantly reduced in children with LN, there was no significant difference in the mean absolute number of activated CD3+CD4−CD8−CD69+ and CD3+CD4−CD8−HLA-DR+ T cells between patients with active LN and inactive LN (p = 0.252 and p = 0.792, respectively), NS (p = 0.150 and p = 0.329, respectively), or HC (p = 0.213 and p = 0.326, respectively).
Double negative T cells and activated double negative T cells in children with active LN, inactive LN, nephrotic syndrome (NS), and healthy controls (HC). a Comparison of the percentage of CD3+CD4−CD8− T cells within CD3+ T cells, b the percentage of CD69 expressing CD3+CD4−CD8− T cells, and c the percentage of HLA-DR expressing CD3+CD4−CD8− T cells within CD3+CD4−CD8− T cells in children with active LN, inactive LN, NS, and HC. Data are expressed as mean ± SEM. p values of <0.05 were considered significant (Mann–Whitney U test)
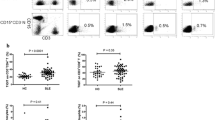
Decrease of Circulating CXCR3 and CCR2 Expressing CD4+ T Cells Associated with High Serum IP10 and CCL2 in Active LN
There was a weak to moderate but significant inverse correlation between the renal SLEDAI-2K and the frequencies and absolute numbers of circulating CD4+CXCR3+ (r = −0.536, p = 0.004 and r = −0.473, p = 0.006, respectively) and CD4+CCR2+ (r = −0.442, p = 0.021 and r = −0.482, p = 0.006, respectively) T cells (Fig. 2a, b) with a mean frequency of 24.8 ± 2.2% for active LN and 36.7 ± 5.1% for inactive LN (Online Resource 4). The highest percentages of CXCR3 and CCR2 expressing CD4+ T cells were observed in patients who were in remission since 1 to 2 1/2 months. Thus, it may be possible that a significant increase in the frequency of circulating CXCR3 and CCR2 expressing CD4+ T cells occurs transiently after achieving remission.
Correlation of CD4+CXCR3+ T cells, CD4+CCR2+ T cells, IP-10, CCL2, and the renal SLEDAI-2K in children with lupus nephritis. a The frequency of circulating CD4+CXCR3+ T cells and b CD4+CCR2+ T cells within CD4+ T cells correlated inversely with the renal SLEDAI-2K (a r = −0.5356, p = 0.0040 and b r = −0.4420, p = 0.0210), whereas the serum concentration of c IP-10 and d CCL2 showed a positive correlation with the renal SLEDAI-2K (c r = 0.6892, p < 0.0001 and d r = 0.7352, p < 0.0001)
The absolute numbers of CD4+CXCR3+ T cells in active LN (124 ± 19/μl) were lower than in inactive LN (184 ± 25/μl) or HC (322 ± 27/μl, p < 0.0001). Also, children with active LN had lower absolute numbers of circulating CD4+CCR2+ T cells (8 ± 4/μl) compared with those with inactive LN (24 ± 7/μl, p = 0.005) or HC (19 ± 2/μl, p = 0.0004).
In contrast, serum IP-10 and CCL2 concentrations correlated positively with the renal SLEDAI-2K (r = 0.689, p < 0.0001 and r = 0.735, p < 0.0001, respectively; Fig. 2c, d). Mean IP10 and CCL2 concentrations were significantly higher in active (361 ± 68 and 318 ± 45 pg/ml, respectively) compared with inactive LN (83 ± 11 pg/ml, p = 0.0002 and 107 ± 9 pg/ml, p = 0.0002, respectively), NS (66 ± 8 pg/ml, p < 0.0001 and 98 ± 13 pg/ml, p < 0.0001, respectively), or HC (69 ± 8 pg/ml, p < 0.0001 and 102 ± 10 pg/ml, p < 0.0001, respectively). However, the percentages of circulating CXCR3+ or CCR2+ expressing CD8+ T cells in active (51.9 ± 5.5% or 5.5 ± 3.7%, respectively) and inactive LN (62.8 ± 3.7% or 4.6% ± 1.8%, respectively), NS (68.9 ± 2.7% or 2.9 ± 1.0%, respectively), and HC (64.2 ± 3.1% or 1.6 ± 0.3%, respectively) were comparable. Neither the frequencies of CXCR3+ nor CCR2+CD8+ T cells correlated with the renal SLEDAI-2K (r = −0.339, p = 0.083 and r = −0.140, p = 0.496, respectively).
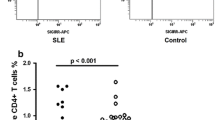
Circulating CD4+Foxp3+ T Cells and TFG-β Levels Inversely Correlate with Serum IL-17 and IL-23 Levels and the Renal SLEDAI-2K in Childhood LN
There was a significant inverse correlation between the frequency of circulating CD4+Foxp3+ T cells and the renal SLEDAI-2K (r = −0.586, p = 0.001). While a significant positive correlation was observed between the serum levels of IL-17 or IL-23 and the renal SLEDAI-2K (r = 0.552, p = 0.003 or r = 0.612, p = 0.0007, respectively), serum TGF-β levels correlated inversely with the renal SLEDAI-2K (r = −0.625, p = 0.0005), and in turn with the concentrations of IL-17 (r = −0.578, p = 0.002) and weakly with IL-23 (r = −0.411, p = 0.033). Further, the frequency of CD4+Foxp3+ T cells inversely correlated with the levels of IL-17 (r = −0.538, p = 0.004) and IL-23 (r = −0.449, p = 0.019), but positively with TGF-β levels (r = 0.360, p = 0.033). The frequencies of CD4+Foxp3+ T cells were significantly lower in active than in inactive LN (p = 0.0084) (Fig. 3a and Online Resource 5). Also, the absolute numbers of CD4+Foxp3+ T cells were significantly lower in the peripheral blood of children with active LN (21 ± 4/μl) than in those with inactive LN (78 ± 32/μl, p = 0.009) or HC (82 ± 22/μl, p = 0.029). Concomitantly, serum TGF-β concentrations were significantly lower in active than in inactive LN (p = 0.0027), NS (42.7 ± 4.6 ng/ml, p = 0.0002), or HC (p < 0.0001) (Fig. 3b). Also, in inactive LN, TGF-β levels were significantly lower than in HC (p = 0.016). On the contrary, serum IL-17 and IL-23 concentrations were significantly higher in active LN compared with inactive LN (p = 0.014 and p = 0.0006, respectively), NS (8.9 ± 3.0 pg/ml, p = 0.0002 and 9.9 ± 2.5 pg/ml, p < 0.0001, respectively), or HC (p < 0.0001 and p < 0.0001, respectively) (Fig. 3c, d). There was no significant difference in the levels of IL-17 and IL-23 between patients with inactive LN and NS (p = 0.217 and p = 0.579, respectively) or HC (p = 0.118 and p = 0.666, respectively).
Decreased frequency of CD4+Foxp3+ T cells and serum TGF-β levels and high serum concentrations of IL-17 and IL-23 in active childhood LN. a The frequency of circulating CD4+Foxp3+ T cells, b serum levels of TGF-β, c IL-17, and d IL-23 in children with active LN and inactive LN and healthy controls. Data are expressed as mean ± SEM. p values of <0.05 were considered significant (Mann–Whitney U test)
Enhanced Th17 and Th1/Th17 Cell Differentiation in Children with LN
While children with LN displayed significantly higher frequencies of Th17 (IL-17+/INF- γ−) cells (10.5 ± 1.0%) and Th1/Th17 (IL-17+/INF- γ+) cells (14.0% ± 1.6%) compared with HC (7.2 ± 1.3%, p = 0.030 and 8.2 ± 0.9%, p = 0.007, respectively), no significant difference in the frequency of Th1 (IL-17−/INF- γ+) cells between children with LN (43.1 ± 3.6%) and HC (37.3 ± 3.7%, p = 0.260) was noted (Fig. 4).
Frequency of IL-17+INF- γ− T cells, IL-17+INF-γ+ T cells, and IL-17−INF-γ+ T cells after stimulation with anti-CD3/anti-CD28 in vitro. Comparison of the percentage of a CD3+IL-17+INF-γ− T cells, b CD3+IL-17+INF-γ+ T cells, and c CD3+IL-17−INF-γ+ T cells within the CD3+ T cell population from children with lupus nephritis (LN) and healthy controls (HC). Data are expressed as mean ± SEM. p values of <0.05 were considered significant (Mann–Whitney U test)
T Cell Phenotype and Medications
It is beyond the scope of this study to determine the specific effects of different immunosuppressive drugs on the T cell phenotype in children with lupus nephritis. It is however important to note, that the frequencies of CD4+CD69+, CD4+HLA-DR+, CD4+CD134+, CD3+CD4−CD8−, CD3+CD4−CD8−CD69+, CD3+CD4−CD8−HLA-DR+, CD4+CXCR3+, CD4+CCR2+, and CD4+Foxp3+ T cells were not significantly different in children with NS treated with prednisone, MMF or cyclosporine and healthy controls (p = 0.558, p = 0.181, p = 0.445, p = 0.969, p = 0.876, p = 0.972, p = 0.487, p = 0.343, and p = 0.061, respectively). However, the frequencies of CD4+CD69+, CD4+HLA-DR+, CD4+CD134+, CD3+CD4−CD8−, CD3+CD4−CD8−CD69+, CD3+CD4−CD8−HLA-DR+, CD4+CXCR3+, CD4+CCR2+, and CD4+Foxp3+ T cells in children with active LN and MMF treatment were significantly different from those in children with NS and MMF therapy (p = 0.007, p = 0.048, p = 0.045, p = 0.028, p = 0.004, p = 0.036, p = 0.024, p = 0.042, and p = 0.034, respectively). The frequencies of DNT cells were lower in children with active LN treated with prednisone (3.1 ± 0.4%) than in those with NS treated with prednisone (5.1 ± 1.3%). Frequencies of CD4+CD69+, CD4+HLA-DR+, CD4+CD134+, CD3+CD4−CD8−CD69+ T cells, and CD3+CD4−CD8−HLA-DR+ were higher in patients with active LN and prednisone treatment (3.1 ± 0.6%, 10.9 ± 2.7%, 0.6 ± 0.3%, 11.5 ± 2.0%, and 24.3 ± 5.9%, respectively) than in those with NS and prednisone (1.7 ± 0.5%, 3.3 ± 0.7%, 0.1 ± 0.0%, 4.7 ± 2.8%, and 5.4 ± 2.5%, respectively).
Although six patients with active LN as well as ten patients with inactive LN had received intravenous pulse cyclophosphamide with a median of 42 months (4–72) and 28 months (3–60), respectively, prior to enrollment into the study, the frequencies of circulating DN T cells, activated DN T cells, CD4+CXCR3+, CD4+CCR2+, CD4+Foxp3+ T cells, and the concentrations of IP-10, CCL2, TGF-β, IL-17, and IL-23 were significantly different between patients with active and inactive disease, as outlined above. All together, these results indicate that the altered T cell phenotypes in active childhood LN as described in this study were not significantly influenced by immunosuppressive medications.
T Cell Phenotype and Age
The frequencies of circulating CD3+CD4−CD8−, CD3+CD4−CD8−CD69+, CD3+CD4−CD8−HLA-DR+, CD4+CD69+, CD4+HLA-DR+, CD4+CD134+, CD8+CD69+, CD8+HLADR+, CD8+CD134+, CD4+CXCR3+, CD4+CCR2+, and CD4+Foxp3+ T cells did not correlate with age in the overall study population (r = −0.193, p = 0.076; r = 0.212, p = 0.066; r = 0.150, p = 0.206; r = 0.223, p = 0.054; r = 0.214, p = 0.070; r = 0.173, p = 0.108; r = 0.203, p = 0.072; r = 0.127, p = 0.180; r = 0.158, p = 0.131; r = 0.084, p = 0.289; r = 0.110, p = 0.216; and r = 0.092, p = 0.270, respectively).
Discussion
In this study, we demonstrated that children with LN displayed profound alterations of the peripheral T cell phenotype, T cell homeostasis, and cytokine milieu during active disease. The frequencies of circulating CD4+CXCR3+, CD4+CCR2+, and CD4+Foxp3+ T cells correlated inversely, while the serum concentrations of IP-10, CCL2, IL-17, and IL-23 correlated positively with the severity of lupus kidney disease activity, as revealed by the renal SLEDAI-2K. Furthermore, following activation, in vitro expansion of pro-inflammatory Th17 and Th1/Th17 cells was significantly increased in children with LN compared with HC.
In addition, our findings suggest continuously increased T cell activity in childhood LN. We show that in children with active and inactive LN, expression of the early activation antigen CD69 on CD4+ T cells and the late T cell activation marker HLA-DR on CD4+ and CD8+ T cells was significantly higher than in controls. Further, we observed a significantly increased frequency of CD134 expressing CD4+ T cells in children with LN as compared to HC. Signaling through the co-stimulatory molecule CD134 that is induced on activated T cells abrogates peripheral T cell tolerance and promotes effector and memory T cell expansion [18]. Moreover, CD134 inhibits the induction of Foxp3 in naive T cells and thus the generation of inducible regulatory T cells [19]. CD134 expression has also recently been related to IL-17-mediated renal inflammation in adults with LN [6]. Our findings suggest that CD134 is involved in pathogenic mechanisms of childhood-onset LN.
Following secondary activation, T cells that constitutively express the chemokine receptor CCR7 differentiate into CCR7− effector memory cells that migrate to inflamed tissue [20]. The critical role of CCR7 in the maintenance of peripheral tolerance has been demonstrated in humans [21] and animal models, showing multi-organ autoimmunity including nephritis in CCR7-deficient mice [22]. We found that children with LN displayed significantly reduced naive CD4+ and CD8+ CD45RA+CCR7+ T cells compared to children with NS or HC, while the frequency of effector memory CD4+ and CD8+CD45RO+ T cells was clearly enhanced, suggesting ongoing antigen driven T cell activation. Moreover, in active childhood LN, effector memory CD4+CD45RO+CCR7− T cells and terminal differentiated CD4+CD45RA+CCR7− T cells increased significantly while central memory CD4+CD45RO+CCR7+ T cells decreased when compared to inactive LN. This indicates further enhanced CD4+ T cell stimulation, subsequent differentiation, and a shift toward an effector T cell phenotype during active disease.
Among the chemokines induced during inflammation that mediate leukocyte trafficking, CCL2 and IP10 and their respective receptors CXCR3 and CCR2 are crucially involved in the autoimmune renal injury of SLE in adults and murine models [7, 23, 24]. CXCR3 has been suggested to have a decisive role in the trafficking of Th1 and Th17 effector T cells into the kidney in murine lupus models [7]. The reduction of peripheral circulating CXCR3 and CCR2 expressing CD4+ T cells in active childhood LN and inverse correlation with the renal SLEDAI-2K as observed in this study may reflect the severity of renal inflammation with high intrarenal concentrations of IP10 and CCL2 and subsequent chemotactic migration of these cells into the renal tissue. Though renal biopsy samples were not available in this study, children with active LN displayed high serum concentrations of IP10 and CCL2, suggesting a critical role of these chemokines in the inflammatory process.
Pro-inflammatory Th17 cells have been shown to induce renal injury in experimental nephritis [25]. Furthermore, double negative T cells known to be pathogenic in adults with SLE produce significant amounts of IL-17, infiltrate the kidneys in LN, and importantly, contribute to renal injury [4]. We show that activated DN T cells are increased in children with active as well inactive LN. While children with inactive LN displayed a high frequency of total circulating DN T cells, we observed a reduction in active childhood LN, suggesting that during active disease, these cells may migrate into the kidney.
Recently, an enormous heterogeneity and functional plasticity of Th cells has been described [26]. Under certain circumstances, the pattern of cytokine secretion and function may switch from that of one Th lineage toward another [26]. While Th17 development is driven by low doses of TGF-β, one of the cytokines implicated in the maintenance of T cell homeostasis [27], high doses inhibit Th17 development and promote regulatory T cells [28]. T regulatory cells in turn can convert into Th17 cells upon activation in an inflammatory cytokine milieu [29]. The terminal differentiation and pathogenic function of Th17 cells however is mediated by IL-23 [30], a cytokine known to play a critical role in the development of LN [31]. Moreover, IL-23 is required for the deviation of Th17 cells toward INF-γ/IL-17 double expressing Th1/Th17 cells [32]. Though Th1 and Th17 cells have the capacity to cause autoimmunity independently [33], recent data suggest that they might collaborate during inflammation and INF-γ/IL-17 double expressing T cells might promote disease progression [33]. Our data show that children with LN displayed in the active state high serum levels of IL-17 and IL-23, while TGF-β was significantly reduced along with reduced circulating regulatory Foxp3+ T cells. TGF-β levels and regulatory T cells correlated inversely with the renal SLEDAI-2K, IL-17, and IL-23 levels, the latter two in turn correlated positively with the renal SLEDAI-2K. Furthermore, following activation, in vitro expansion of pro-inflammatory Th17 and Th1/Th17 cells in children with LN was significantly greater than in HC. This imbalance of inflammatory and immunoregulatory cytokines may importantly contribute to the development and progression of the autoimmune process in childhood LN.
Due to the enormous complexity of mechanisms involved in the pathogenesis of LN, the design of future treatments that can effectively interrupt pathogenic mechanisms seems difficult. However, due to the far-reaching interrelations of immune mechanisms, the plasticity of certain T cell subsets, and the known links between major pathogenic mechanisms of LN involving CXCR3, CCR2, DN T cells, regulatory T cells, Th17 cells, IL-23, and TGF-β, therapeutic regulation of the aberrant cytokine expression might reverse the autoimmune state into a state of immune tolerance. Further investigations are needed to define the impact of cytokine modulation on the immuno-pathomechanisms of childhood LN and possible therapeutic benefits.
Conclusions
Active disease in children with LN is characterized by high levels of the pro-inflammatory cytokines IP-10, CCL2, IL-17, and IL-23 and by an increase of effector CD4+CD45RO+CCR7−, Th17, and Th1/Th17 cells along with significantly reduced concentrations of TGF-β and Foxp3+ regulatory T cells. Therapeutic down-regulation of these pro-inflammatory cytokines may specifically suppress lupus nephritis activity in children without interfering with the function of the protective immune system.
References
Crispin JC, Tsokos GC. Novel molecular targets in the treatment of systemic lupus erythematosus. Autoimmun Rev. 2008;7(3):256–61. doi:10.1016/j.autrev.2007.11.020.
Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990;37(1):100–9.
Crispin JC, Kyttaris VC, Juang YT, Tsokos GC. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 2008;29(3):110–5.
Crispin JC, Oukka M, Bayliss G, Cohen RA, Van Beek CA, Stillman IE, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181(12):8761–6.
Rajagopalan S, Zordan T, Tsokos GC, Datta SK. Pathogenic anti-DNA autoantibody-inducing T helper cell lines from patients with active lupus nephritis: isolation of CD4-8− T helper cell lines that express the gamma delta T-cell antigen receptor. Proc Natl Acad Sci U S A. 1990;87(18):7020–4.
Dolff S, Quandt D, Wilde B, Feldkamp T, Hua F, Cai X, et al. Increased expression of costimulatory markers CD134 and CD80 on interleukin-17 producing T cells in patients with systemic lupus erythematosus. Arthritis Res Ther. 12(4):R150. doi:10.1186/ar3100.
Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183(7):4693–704. doi:10.4049/jimmunol.0802626.
Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–87. doi:10.1016/j.cell.2008.05.009.
Humrich JY, Morbach H, Undeutsch R, Enghard P, Rosenberger S, Weigert O, et al. Homeostatic imbalance of regulatory and effector T cells due to IL-2 deprivation amplifies murine lupus. Proc Natl Acad Sci U S A. 107(1):204–9. doi:10.1073/pnas.0903158107.
La Cava A. The busy life of regulatory T cells in systemic lupus erythematosus. Discov Med. 2009;8(40):13–7.
Brunner HI, Gladman DD, Ibanez D, Urowitz MD, Silverman ED. Difference in disease features between childhood-onset and adult-onset systemic lupus erythematosus. Arthritis Rheum. 2008;58(2):556–62.
Bartosh SM, Fine RN, Sullivan EK. Outcome after transplantation of young patients with systemic lupus erythematosus: a report of the North American pediatric renal transplant cooperative study. Transplantation. 2001;72(5):973–8.
Doria A, Vesco P, Zulian F, Gambari PF. The 1982 ARA/ACR criteria for the classification of systemic lupus erythematosus in pediatric and adult patients. Clin Exp Rheumatol. 1994;12(6):689–90.
Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725. doi:10.1002/1529-0131(199709)40:9<1725::AID-ART29>3.0.CO;2-Y.
Weening JJ, D’Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241–50.
Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288–91.
Brunner HI, Feldman BM, Bombardier C, Silverman ED. Sensitivity of the Systemic Lupus Erythematosus Disease Activity Index, British Isles Lupus Assessment Group Index, and Systemic Lupus Activity Measure in the evaluation of clinical change in childhood-onset systemic lupus erythematosus. Arthritis Rheum. 1999;42(7):1354–60. doi:10.1002/1529-0131(199907)42:7<1354::AID-ANR8>3.0.CO;2-4.
Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229(1):173–91. doi:10.1111/j.1600-065X.2009.00766.x.
Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, et al. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–10. doi:10.1182/blood-2007-01-070748.
Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–12. doi:10.1038/44385.
Worbs T, Forster R. A key role for CCR7 in establishing central and peripheral tolerance. Trends Immunol. 2007;28(6):274–80. doi:10.1016/j.it.2007.04.002.
Davalos-Misslitz AC, Rieckenberg J, Willenzon S, Worbs T, Kremmer E, Bernhardt G, et al. Generalized multi-organ autoimmunity in CCR7-deficient mice. Eur J Immunol. 2007;37(3):613–22. doi:10.1002/eji.200636656.
Kulkarni O, Anders HJ. Chemokines in lupus nephritis. Front Biosci. 2008;13:3312–20.
El-Shehaby A, Darweesh H, El-Khatib M, Momtaz M, Marzouk S, El-Shaarawy N, et al. Correlations of urinary biomarkers, TNF-like weak inducer of apoptosis (TWEAK), osteoprotegerin (OPG), monocyte chemoattractant protein-1 (MCP-1), and IL-8 with lupus nephritis. J Clin Immunol. 2011;31(5):848–56. doi:10.1007/s10875-011-9555-1.
Summers SA, Steinmetz OM, Li M, Kausman JY, Semple T, Edgtton KL, et al. Th1 and Th17 cells induce proliferative glomerulonephritis. J Am Soc Nephrol. 2009;20(12):2518–24. doi:10.1681/ASN.2009030337.
Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol. 2009;21(3):274–80. doi:10.1016/j.coi.2009.05.021.
Gorelik L, Flavell RA. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12(2):171–81.
Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–9. doi:10.1038/ni.1610.
Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178(11):6725–9.
McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10(3):314–24. doi:10.1038/ni.1698.
Zhang Z, Kyttaris VC, Tsokos GC. The role of IL-23/IL-17 axis in lupus nephritis. J Immunol. 2009;183(5):3160–9. doi:10.4049/jimmunol.0900385.
Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12(3):255–63. doi:10.1038/ni.1993.
Damsker JM, Hansen AM, Caspi RR. Th1 and Th17 cells: adversaries and collaborators. Ann N Y Acad Sci. 2010;1183:211–21. doi:10.1111/j.1749-6632.2009.05133.x.
Acknowledgments
This work was supported by OeNB Jubiläumsfonds Grant (13334) and Medizinischer Forschungsfonds Tirol grant to ME.
Disclosures
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is dedicated in memory of Prof. Dr. Lothar Bernd Zimmerhackl.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
Renal SLEDAI-2K, total SLEDAI-2K, and extra-renal manifestations of SLE at the time of sampling in children with active LN (PDF 56 kb)
Online Resource 2
Representative FACS dot plots showing the frequencies of circulating CD45RA+CCR7− (A1), CD45RA+CCR7+ (A2), CD45RA−CCR7− (A3), and CD45RA−CCR7+ (A4) cells on gated CD4+ T cells from a patient with active lupus nephritis (16.9%, 29.4%, 34.5%, and 19.2%, respectively), inactive lupus nephritis (12.7%, 39.4%, 27.5%, and 20.4%, respectively), and a control subject (5.2%, 47.4%, 22.1%, and 25.3%, respectively) (PDF 158 kb)
Online Resource 3
Increased frequency of CD69 expressing DNT cells in childhood LN. Leukocytes were stained with monoclonal antibodies to CD16/56, CD3, CD4, CD8, and CD69. Representative FACS dot plots show b) the frequencies of CD3+CD4−CD8− T cells (DNT) on gated CD3+CD16/56− cells, numbers indicate the percentage of CD3+CD4−CD8− cells and c expression of CD69 on gated DNT cells, numbers indicate the percentage of double positive cells (PDF 1.30 mb)
Online Resource 4
Reduced circulating CD4+CXCR3+ T cells in active childhood-onset LN. Representative FACS dot plots of CXCR3 expression in gated CD4+ T cells from a child with active LN, inactive LN, nephrotic syndrome (NS), and a control subject. Numbers indicate the percentage of double positive cells (PDF 493 kb)
Online Resource 5
Decrease of circulating CD4+Foxp3+ T cells in active childhood-onset LN. Representative examples of Foxp3 expression in circulating CD4+ T cells of a patient with active LN, inactive LN, nephrotic syndrome (NS), and a control subject. Numbers indicate the percentage of double positive cells (PDF 245 kb)
Rights and permissions
About this article
Cite this article
Edelbauer, M., Kshirsagar, S., Riedl, M. et al. Activity of Childhood Lupus Nephritis is Linked to Altered T Cell and Cytokine Homeostasis. J Clin Immunol 32, 477–487 (2012). https://doi.org/10.1007/s10875-011-9637-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9637-0