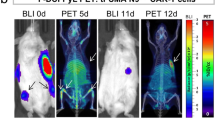
Abstract
Adoptive immunotherapy using chimeric antigen receptor-engrafted T cells is a promising emerging therapy for cancer. Prior to clinical testing, it is mandatory to evaluate human therapeutic cell products in meaningful in vivo pre-clinical models. Here, we describe the use of fused single-photon emission CT–CT imaging to monitor real-time migration of chimeric antigen receptor-engineered T cells in immune compromised (SCID Beige) mice. Following intravenous administration, human T cells migrate in a highly similar manner to that reported in man, but penetrate poorly into established tumors. By contrast, when delivered via intraperitoneal or subcutaneous routes, T cells remain at the site of inoculation with minimal systemic absorption—irrespective of the presence or absence of tumor. Together, these data support the validity of pre-clinical testing of human T-cell immunotherapy in SCID Beige mice. In light of their established efficacy, regional administration of engineered human T cells represents an attractive therapeutic option to minimize toxicity in the treatment of selected malignancies.




Similar content being viewed by others
References
Sprangers B, Van Wijmeersch B, Fevery S, Waer M, Billiau AD. Experimental and clinical approaches for optimization of the graft-versus-leukemia effect. Nat Clin Pract Oncol. 2007;4:404–14.
Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–40.
Schmitt TM, Ragnarsson GB, Greenberg PD. T cell receptor gene therapy for cancer. Hum Gene Ther. 2009;20:1240–8.
Sadelain M. T-cell engineering for cancer immunotherapy. Cancer J. 2009;15:451–5.
Davies DM, Maher J. Adoptive T-cell immunotherapy of cancer using chimeric antigen receptor-grafted T-cells. Arch Immunol Ther Exp. 2010;58:165–78.
Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, et al. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008;14:1390–5.
Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–36.
Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nat Biotechnol. 2002;20:70–5.
Pulè MA, Straathof KC, Dotti G, Heslop HE, Rooney CM, Brenner MK. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther. 2005;12:933–41.
Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D, et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther. 2009;17:1453–64.
Murphy WJ, Tian ZG, Asai O, Funakoshi S, Rotter P, Henry M, et al. Chemokines and T lymphocyte activation: II. Facilitation of human T cell trafficking in severe combined immunodeficiency mice. J Immunol. 1996;156:2104–11.
Taub DD, Tsarfaty G, Lloyd AR, Durum SK, Longo DL, Murphy WJ. Growth hormone promotes human T cell adhesion and migration to both human and murine matrix proteins in vitro and directly promotes xenogeneic engraftment. J Clin Invest. 1994;94:293–300.
Nervi B, Rettig MP, Ritchey JK, Wang HL, Bauer G, Walker J, et al. Factors affecting human T cell engraftment, trafficking, and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp Hematol. 2007;35:1823–38.
Santos EB, Yeh R, Lee J, Nikhamin Y, Punzalan B, Punzalan B, et al. Sensitive in vivo imaging of T cells using a membrane-bound Gaussia princeps luciferase. Nat Med. 2009;15:338–44.
Dobrenkov K, Olszewska M, Likar Y, Shenker L, Gunset G, Cai S, et al. Monitoring the efficacy of adoptively transferred prostate cancer-targeted human T lymphocytes with PET and bioluminescence imaging. J Nucl Med. 2008;49:1162–70.
Brown CE, Vishwanath RP, Aguilar B, Starr R, Najbauer J, Aboody KS, et al. Tumor-derived chemokine MCP-1/CCL2 is sufficient for mediating tumor tropism of adoptively transferred T cells. J Immunol. 2007;179:3332–41.
Wilkie S, Picco G, Foster J, Davies DM, Julien S, Cooper L, et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. J Immunol. 2008;180:4901–9.
Davies DM, Wilkie S, Foster JM, Delinassios G, Chiapero-Stanke L, Burbridge S, et al. Targeting the extended Erbb receptor family using chimeric antigen receptor (CAR)-grafted T-cells as a treatment for head and neck cancer. In: Proceedings of the 101st Annual Meeting of the American Association for Cancer Research, AACR, Washington, DC, USA, 17–21 April 2010. Abstract no. 1932.
Pittet MJ, Grimm J, Berger CR, Tamura T, Wojtkiewicz G, Nahrendorf M, et al. In vivo imaging of T cell delivery to tumors after adoptive transfer therapy. Proc Natl Acad Sci USA. 2007;104:12457–61.
Wingens M, Walma T, van Ingen H, Stortelers C, van Leeuwen JE, van Zoelen EJ, et al. Structural analysis of an epidermal growth factor/transforming growth factor-alpha chimera with unique ErbB binding specificity. J Biol Chem. 2003;278:39114–23.
Wilkie S, Burbridge SE, Chiapero-Stanke L, Pereira AC, Cleary S, van der Stegen SJ, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem. 2010;285:25538–44.
Read EJ, Keenan AM, Carter CS, Yolles PS, Davey RJ. In vivo traffic of indium-111-oxine labelled human lymphocytes collected by automated apheresis. J Nucl Med. 1990;31:999–1006.
Wagstaff J, Gibson C, Thatcher N, Ford WL, Sharma H, Crowther D. Human lymphocyte traffic assessed by indium 111oxine labelling: clinical observations. Clin Exp Immunol. 1981;43:443–9.
Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, et al. Tumor localization of adoptively transferred indium-111 labelled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–61.
Smith ME, Ford WL. The recirculating lymphocyte pool of the rat: a systematic description of the migratory behaviour of recirculating lymphocytes. Immunology. 1983;49:83–94.
Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. Stabilized imaging of immune surveillance in the mouse lung. Nat Methods. 2011;8:91–6.
Hamann A, Klugewitz K, Austrup F, Jablonski-Westrich D. Activation induces rapid and profound alterations in the trafficking of T cells. Eur J Immunol. 2000;30:3207–18.
Staunton DE, Dustin ML, Erickson HP, Springer TA. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990;61:243–54.
Aird WC. Phenotypic heterogeneity of the Endothelium. 1. Structure, Function and Mechanisms. Circ Res. 2007;100:158–73.
Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–51.
Heslop HE. Safer CARs. Mol Ther. 2010;18:661–2.
Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R, et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–2.
Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nährig J, et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother. 2008;57:271–80.
Koya RC, Mok S, Comin-Anduix B, Chodon T, Radu CG, Nishimura MI, et al. Kinetic phases of distribution and tumor targeting by T cell receptor engineered lymphocytes inducing robust antitumor responses. Proc Natl Acad Sci USA. 2010;107:14286–91.
Brentjens R, Yeh R, Bernal Y, Riviere I, Sadelain M. Treatment of chronic lymphocytic leukemia with genetically targeted autologous T cells: case report of an unforeseen adverse event in a phase I clinical trial. Mol Ther. 2010;18:666–8.
Crispe IN, Dao T, Klugewitz K, Mehal WZ, Metz DP. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62.
Kershaw MH, Wang G, Westwood JA, Pachynski RK, Tiffany HL, Marincola FM, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13:1971–80.
Lo AS, Taylor JR, Farzaneh F, Kemeny DM, Dibb NJ, Maher J. Harnessing the tumour-derived cytokine, CSF-1, to co-stimulate T-cell growth and activation. Mol Immunol. 2008;45:1276–87.
Schliemann C, Palumbo A, Zuberbühler K, Villa A, Kaspar M, Trachsel E, et al. Complete eradication of human B-cell lymphoma xenografts using rituximab in combination with the immunocytokine L19-IL2. Blood. 2009;113:2275–83.
Cappuccini F, Lucci 3rd JA, Dett CA, Gatanaga M, Ininns EK, Gatanaga T, et al. Trafficking of syngeneic murine lymphokine activated killer T cells following intraperitoneal administration in normal and tumor bearing mice. Gynecol Oncol. 1992;46:163–9.
Markman M. Intraperitoneal chemotherapy as primary treatment of advanced ovarian cancer: efficacy, toxicity, and future directions. Rev Recent Clin Trials. 2007;2:169–73.
Van Elssen CH, Frings PW, Bot FJ, Van de Vijver KK, Huls MB, Meek B, et al. Expression of aberrantly glycosylated Mucin-1 in ovarian cancer. Histopathology. 2010;57:597–606.
Simpson BJ, Phillips HA, Lessells AM, Langdon SP, Miller WR. c-erbB growth-factor-receptor proteins in ovarian tumours. Int J Cancer. 1995;64:202–6.
Acknowledgements
We are very grateful to Dr Joy Burchell and Prof Joyce Taylor-Papadimitriou for provision of several highly useful MUC1-related reagents. This work was supported by the US Department of Defense (Fiscal Year 2008 Ovarian Cancer Research Program, Translational Research Partnership Award) under contract W81XWH-09-1-0096; Breast Cancer Campaign (project grant 2006NovPR18), Association for International Cancer Research (project grant 08-0419), Guy’s and St Thomas’ Charity, Experimental Cancer Medicine Centre (King’s College London) and from Guy’s and the Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parente-Pereira, A.C., Burnet, J., Ellison, D. et al. Trafficking of CAR-Engineered Human T Cells Following Regional or Systemic Adoptive Transfer in SCID Beige Mice. J Clin Immunol 31, 710–718 (2011). https://doi.org/10.1007/s10875-011-9532-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9532-8