Abstract
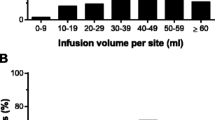
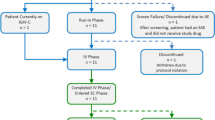
A multi-center, prospective, open-label study was conducted in primary immunodeficiency disease patients to determine the tolerability and pharmacokinetics of a 10% liquid IgG preparation administered subcutaneously. Forty-nine subjects (3–77 years old) were enrolled. Pharmacokinetic equivalence of subcutaneous treatment was achieved at a median dose of 137% of the intravenous dose, with a mean trough IgG level of 1,202 mg/dL at the end of the assessment period. The overall infection rate during subcutaneous treatment was 4.1 per subject-year. Three acute serious bacterial infections were reported, resulting in a rate of 0.067 per subject-year. A low overall rate of temporally associated adverse events (8%), and a very low rate of infusion site adverse events (2.8%), was seen at volumes up to 30 mL/site and rates ≤30 mL/h/site. Thus, subcutaneous replacement therapy with a 10% IgG preparation proved effective, safe and well-tolerated in our study population of subjects with primary immunodeficiency disease.


Similar content being viewed by others
References
Gardulf A. Immunoglobulin treatment for primary antibody deficiencies. Advantages of the subcutaneous route. Biodrogs. 2007;21(2):105–16.
Chapel HM, Spickett GP, Ericson D, Engl W, Eibl MM, Björkander J. The comparison of the efficacy and safety of intravenous versus subcutaneous immunoglobulin replacement therapy. J Clin Immunol. 2000;20(2):94–100.
Berger M. A history of immune globulin therapy, from the Harvard crash program to monoclonal antibodies. Curr Allergy Asthma Rep. 2002;2(5):368–78.
Ochs HD, Gupta S, Kiessling P, et al. Safety and efficacy of self-administered subcutaneous immunoglobulin in patients with primary immunodeficiency diseases. J Clin Immunol. 2006;26:265–73.
Gardulf A, Nicolay U, Asensio O, et al. Rapid subcutaneous IgG replacement therapy is effective and safe in children and adults with primary immunodeficiencies—A prospective multi-national study. J Clin Immunol. 2006;26:177–85.
Radinsky S, Bonagura VR. Subcutaneous immunoglobulin infusion as an alternative to intravenous immunoglobulin. J Allergy Clin Immunol. 2003;112(3):630–3.
Roord JJ, van der Meer JW, Kuis W, et al. Home treatment in patients with antibody deficiency by slow subcutaneous infusion of gammaglobulin. Lancet. 1982;1:689–90.
Nicolay U, Kiessling P, Berger M, et al. Health-related quality of life and treatment satisfaction in North American patients with primary immunedeficiency diseases receiving subcutaneous IgG self-infusions at home. J Clin Immunol. 2006;26(1):65–72.
Gardulf A, Nicolay U, Asensio O, et al. Children and adults with primary antibody deficiencies gain quality of life by subcutaneous IgG self-infusions at home. J Allergy Clin Immunol. 2004;114(4):936–42.
Hansen S, Gustafson R, Smith CIE, Gardulf A. Express subcutaneous IgG infusions: decreased time of delivery with maintained safety. Clin Immmunol. 2002;104(3):237–41.
Fasth A, Nyström J. Quality of life and health-care resource utilization among children with primary immunodeficiency receiving home treatment with subcutaneous human immunoglobulin. J Clin Immunol. 2008;28(4):370–8.
IUIS Scientific Committee. Primary immunodeficiency diseases: report of an IUIS Scientific Committee. Clin Exp Immunol. 1999;118 Suppl 1:1–28.
Dette H, Pilz KF. A comparative study of monotone nonparametric kernel estimates. J Statist Comp Simul. 2006;76:41–56.
Food and Drug Administration and Center for Biologics Evaluation and Research. Guidance for industry: safety, efficacy, and pharmacokinetic studies to support marketing of immune globulin intravenous (human) as replacement therapy for primary humoral immunodeficiency (Draft Guidance). 2005. U.S. Department of Health and Human Services, Food and Drug Administration (FDA).
Wasserman RL. Pharmacokinetics and safety of subcutaneous immune globulin (human), 10% caprylate/chromatography purified in patients with primary immunodeficiency disease. Clin Exp Immunol. 2010;161:518–26.
Hagan J. Efficacy and safety of a new 20% immunoglobulin preparation for subcutaneous administration, IgPro20 in patients with primary immunodeficiency. J Clin Immunol. 2010;30:734–45.
Church JA, Leibl H, Stein MR, et al. Efficacy, safety and tolerability of a new 10% liquid intravenous immune globulin (IGIV 10%) in patients with primary immunodeficiency. J Clin Immunol. 2006;26(4):388–95.
Stein MR, Nelson RP, Church JA, et al. Safety and efficacy of privigen®, a novel 10% liquid immunoglobulin preparation for intravenous use, in patients with primary immunodeficiencies. J Clin Immunol. 2009;29(1):137–44.
Björkander J, Nikoskelainen J, Leibl H, et al. Prospective open-labeled study of pharmacokinetics, efficacy and safety of a new 10% liquid intravenous immunoglobulin in patients with hypo- or agammaglobulinemia. Vox Sang. 2006;90:286–93.
Roifman CM, Schroeder H, Berger M, et al. Comparison of the efficacy of IGIV-C, 10% (caprylate/chromatography) and IGIV-SD, 10% as replacement therapy in primary immune deficiency. A randomized double-blind trial. Int Immunopharmacol. 2003;3(9):1325–33.
Ochs HD, Pinciaro PJ, and the Octagam Study Group. Octagam 5%, an intravenous IgG product, is efficacious and well tolerated in subjects with primary immunodeficiency disease. J Clin Immunol. 2004;24(3):309–14.
Eijkhout HW, van der Meer JWM, Kallenberg CGM, Weening RS, van Dissel JT, Sanders LAM, et al. The effect of two different dosages of intravenous immunoglobulin on the incidence of recurrent infections in patients with primary hypogammaglobulinemia. Ann Intern Med. 2001;135(3):165–74.
Orange J, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137(1):21–30.
Siegel J. The product: all intravenous immunoglobulin are not equivalent. Pharmacotherapy. 2005;25(11 pt 2):78S–84.
Gelfand EW. Differences between IGIV products: impact on clinical outcomes. Int Immunopharmacol. 2006;6(4):592–9.
Octapharma AG - Update: Product Withdrawal/Product Recall - Octagam (Octagam 50 mg/ml) and Octagam 10% (Octagam 100 mg/ml). Octapharma AG, 2010. http://www.octapharma.com/about-octapharma/news-events/news-single-view/news/update-product-withdrawalproduct-recall-octagam-octagam-50mgml-and-octagam-10-octagam-100mg.html. Last accessed 23 NOV 2010.
CSL Behring. Vivaglobin. Immune Globulin Subcutaneous (Human), US Label. April 2007.
Acknowledgments
The authors thank the IGSC, 10% Study Group (Rebecca Buckley, Duke University Medical Center, Durham, NC 27710; Melvin Berger, formerly at University Hospitals of Cleveland, Cleveland, OH 44106; Andrew J. Grant, University of Texas Medical Branch, Galveston, TX 77555–1083; Robert L. Roberts, UCLA Medical Center Department of Pediatrics, Los Angeles, CA 90095; John M. Routes, Medical College of Wisconsin, Milwaukee, WI 53226–4874) for their contribution to this study. The authors also thank the following persons from Baxter Innovations GmbH (Vienna, Austria) and Baxter Healthcare Corporation (Westlake Village, CA, USA) for their support in study management, data management or manuscript review: Elizabeth Flax, Sandra Parsons, Smita Barua, Darin Dobler, Carmit Strauss.
Conflicts of Interest
Richard L. Wasserman has served as investigator and consultant for Baxter BioScience, CSL Behring, NABI, Omrix, Talecris. Isaac Melamed, Lisa Kobrynski, Steven D. Strausbaugh, and Mark Stein have served as investigators for Baxter BioScience. Marlies Sharkhawy, Werner Engl, Heinz Leibl, Luba Sobolevsky, David Gelmont, Richard I. Schiff and William J. Grossman are employees of Baxter Healthcare Corporation or Baxter Innovations GmbH.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wasserman, R.L., Melamed, I., Kobrynski, L. et al. Efficacy, Safety, and Pharmacokinetics of a 10% Liquid Immune Globulin Preparation (GAMMAGARD LIQUID, 10%) Administered Subcutaneously in Subjects with Primary Immunodeficiency Disease. J Clin Immunol 31, 323–331 (2011). https://doi.org/10.1007/s10875-011-9512-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-011-9512-z