Abstract
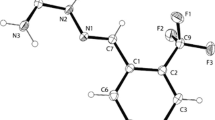
A new semicarbazone, HL has been synthesized from quinoline-2-carboxaldehyde and N4-phenyl-3-semicarbazide and structurally and spectrochemically characterized. 1H NMR, 13C NMR, IR and electronic spectra of the compound are studied. The existence of keto form in the solid state is supported by the crystal structure and IR data. The compound crystallizes into an orthorhombic space group P212121. Intra and intermolecular hydrogen bonding interactions facilitates unit cell packing in the crystal lattice.
Index Abstract
A new semicarbazone, HL has been synthesized from quinoline-2-carboxaldehyde and N4-phenyl-3-semicarbazide and it crystallizes into an orthorhombic with a space group P212121. Unit cell parameters a = 6.4662(3) Å, b = 10.3994(5) Å, c = 21.0315(11) Å, α = 90°, β = 90° and γ = 90°. 1H NMR, 13C NMR, IR and electronic spectra of the compound were studied. The existence of keto form in the solid state is supported by the crystal structure and IR data. Intra and intermolecular hydrogen bonding interactions facilitates unit cell packing in the crystal lattice.









Similar content being viewed by others
References
Casas JS, Garcia-Tasende MS, Sordo J (2000) Coord Chem Rev 209:197
Pandeya SN, Dimmock JP (1993) Pharmazie 48:659
Shalini M, Yogeeswari P, Sriram D, Stables JP (2007) Biomed Pharmacother 8:1
Eisenbraun EJ, Wesley RP, Budhram RS, DewPrasad B (1989) Chem Ind (London) 15:459
Petering HG, VanGiessen GJ (1965) Biochem Copper Proc Symp. Harriman, New York, p 197
West DX, Sonawane PB, Kumbhar AS, Yerande RG (1993) Coord Chem Rev 123:49
Sheldrick GM (1997) SHELXL97 and SHELXS97. University of Göttingen, Germany
Flack HD, Bernardinelli G (2000) J Appl Cryst 33:114
Brandenburg K (2006), Diamond version 3.1d. Crystal Impact GbR, Bonn, Germany
Spek AL (2003) J Appl Cryst 36:7
March J (1992) Advanced organic chemistry, reactions, mechanisms and structure, 4th edn. Wiley, New York
Reena TA, Seena EB, Kurup MRP (2008) Polyhedron 27:1825
Kala UL, Suma S, Kurup MRP, Krishnan Suja, John RP (2007) Polyhedron 26:1427
Seena EB, Kurup MRP, Suresh E (2008) J Chem Cryst 38:93
Manoj E, Kurup MRP, Fun H-K (2008) J Chem Cryst 38:157
Swearingen JK, Kaminsky W, West DX (2002) Trans Met Chem 27:724
Suni V, Nethaji M, Kurup MRP (2005) J Mol Struct 749:177
Joseph M, Suni V, Nayar Chandini R, Kurup MRP, Fun H-K (2004) J Mol Struct 705:63
Reena TA, Seena EB, Kurup MRP (2008) Polyhedron 27:3461
Mayer R (1967) In: Jansen M (ed) Organosulfur Chemistry. Inter-science, New York
Philip V, Suni V, Kurup MRP, Nethaji M (2005) Polyhedron 24:1133
Majumder A, Rosair GM, Mallick A, Chattopadhyay N, Mitra S (2006) Polyhedron 25:1753
Acknowledgements
M.R.P. Kurup is thankful to the Council of Scientific & Industrial Research, New Delhi, India for the financial assistance. The authors are thankful to the National Single Crystal X-ray diffraction Facility, IIT, Bombay, India for providing single crystal XRD data.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reena, T.A., Kurup, M.R.P. Synthesis, Spectral and Structural Studies of a Novel Semicarbazone Synthesized from Quinoline-2-Carboxaldehyde and N4-Phenyl-3-Semicarbazide. J Chem Crystallogr 40, 927–932 (2010). https://doi.org/10.1007/s10870-010-9765-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10870-010-9765-z