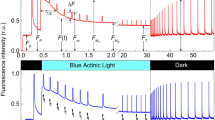
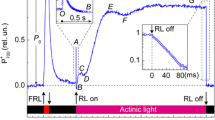
Abstract
Chlorophyll fluorescence induction curves induced by an actinic pulse of red light follow different kinetics in dark-adapted plant leaves and leaves preilluminated with far-red light. This influence of far-red light was abolished in leaves infiltrated with valinomycin known to eliminate the electrical (Δφ) component of the proton-motive force and was strongly enhanced in leaves infiltrated with nigericin that abolishes the ΔpH component. The supposed influence of ionophores on different components of the proton motive force was supported by differential effects of these ionophores on the induction curves of the millisecond component of chlorophyll delayed fluorescence. Comparison of fluorescence induction curves with the kinetics of P700 oxidation in the absence and presence of ionophores suggests that valinomycin facilitates a build-up of a rate-limiting step for electron transport at the site of plastoquinone oxidation, whereas nigericin effectively removes limitations at this site. Far-red light was found to be a particularly effective modulator of electron flows in chloroplasts in the absence of ΔpH backpressure on operation of the electron-transport chain.
Similar content being viewed by others
References
Allen JF (2003) Cyclic, pseudocyclic and noncyclic photophosphorylation: new links in the chain. Trends Plant Sci 8:15–19
Altea F, Stengel A, Benza JP, Petersena E, Soll J, Groll M, Bölter B (2010) Ferredoxin:NADPH oxidoreductase is recruited to thylakoids by binding to a polyproline type II helix in a pH-dependent manner. Proc Natl Acad Sci USA 107:19260–19265
Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30:1107–1125
Braun NA, Davis AW, Theg SM (2007) The chloroplast Tat pathway utilizes the transmembrane electric potential as an energy source. Biophys J 93:1993–1998
Buchta J, Grabolle M, Dau H (2007) Photosynthetic dioxygen formation studied by time-resolved delayed fluorescence measurements. Biochim Biophys Acta 1767:565–574
Bulychev AA (1984) Different kinetics of membrane potential formation in dark-adapted and preilluminated chloroplasts. Biochim Biophys Acta 766:647–652
Bulychev AA (2011) Induction changes in photosystems I and II in plant leaves upon modulation of membrane ion transport. Biochem (Mosc) Suppl Ser A Membr Cell Biol 5:335–342
Bulychev AA, Vredenberg WJ (2001) Modulation of photosystem II chlorophyll fluorescence by electrogenic events generated by photosystem I. Bioelectrochemistry 54:157–168
Bulychev AA, Vredenberg WJ (2010) Induction kinetics of photosystem I-activated P700 oxidation in plant leaves and their dependence on pre-energization. Russ J Plant Physiol 57:599–608
Bulychev AA, Niyazova MM, Turovetsky VB (1985) Evidence for the delayed photoactivation of electrogenic electron transport in chloroplast membranes. Biochim Biophys Acta 808:186–191
Dau H, Sauer K (1991) Electric field effect on chlorophyll fluorescence and its relation to Photosystem II charge separation reactions studied by a salt-jump tecgnique. Biochim Biophys Acta 1098:49–60
Dilley RA (1991) Energy coupling in chloroplasts: a calcium-gated switch controls proton fluxes between localized and delocalized proton gradients. Curr Topics Bioenerg 16:265–318
Feild TS, Nedbal L, Ort DR (1998) Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration. Plant Physiol 116:1209–1218
Foyer C, Furbank R, Harbinson J, Horton P (1990) The mechanisms contributing to photosynthetic control of electron transport by carbon assimilation in leaves. Photosynth Res 25:83–100
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63:1637–1661
Goltsev V, Zaharieva I, Chernev P, Strasser RJ (2009) Delayed fluorescence in photosynthesis. Photosynth Res 101:217–232
Johnson MP, Ruban AV (2011) Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced ΔpH. J Biol Chem 286:19973–19981
Johnson MP, Zia A, Ruban AV (2012) Elevated ΔpH restores rapidly reversible photoprotective energy dissipation in Arabidopsis chloroplasts deficient in lutein and xanthophyll cycle activity. Planta 235:193–204
Joliot P, Johnson GN (2011) Regulation of cyclic and linear electron flow in higher plants. Proc Natl Acad Sci USA 108:13317–13322
Joly D, Jemâa E, Carpentier R (2010) Redox state of the photosynthetic electron transport chain in wild-type and mutant leaves of Arabidopsis thaliana: impact on photosystem II fluorescence. J Photochem Photobiol B Biol 98:180–187
Karlish SJD, Avron M (1971) Energy transfer inhibition and ion movements in isolated chloroplasts. Eur J Biochem 20:51–57
Katona E, Neimanis S, Schönknecht G, Heber U (1992) Photosystem I-dependent cyclic electron transport is important in controlling Photosystem II activity in leaves under conditions of water stress. Photosynth Res 34:449–464
Kramer DM, Crofts AR (1996) Control and measurement of photosynthetic transport in vivo. In: Baker NR (ed) Photosynthesis and environment. Springer, Dordrecht, pp 25–66
Kramer DM, Avenson TJ, Edwards GE (2004) Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci 9:349–357
Lazar D, Schansker G (2009) Models of chlorophyll a fluorescence transents. In: Laisk A, Nedbal L, Govindjee (eds) Photosynthesis in silico. Springer, Dordrecht, pp 85–123
Nixon PJ, Rich PR (2006) Chlororespiratory pathways and their physiological significance. In: Wise RR, Hoober JK (eds) The structure and function of plastids. Springer, Dordrecht, pp 237–251
Renger G (1972) The action of 2-anilinothiophenes as accelerators of the deactivation reactions in the water-splitting enzyme system of photosynthesis. Biochim Biophys Acta 256:428–439
Satoh K (1982) Mechanism of photoactivation of electron transport in intact Bryopsis chloroplasts. Plant Physiol 70:1413–1416
Schansker G, Strasser RJ (2005) Quantification of non-QB-reducing centers in leaves using a far-red pre-illumination. Photosynth Res 84:145–151
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta 1797:1313–1326
Tyystjarvi E, Vass I (2004) Light emission as a probe of charge separation and recombination in the photosynthetic apparatus: Relation of prompt fluorescence to delayed light emission and thermoluminescence. In: Papageorgiou G (ed) Chlorophyll fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 363–388
Voorthuysen T, Bulychev AA, Dassen JНА, Snel JFH, Vredenberg WJ (1996) Suppression of flash-induced PSII-dependent electrogenesis caused by proton pumping in chloroplasts. Physiol Plant 98:156–164
Vredenberg WJ (2011) Kinetic analyses and mathematical modeling of primary photochemical and photoelectrochemical processes in plant photosystems. Biosystems 103:138–151
Vredenberg WJ, Bulychev AA (1976) Changes in the electrical potential across the thylakoid membranes of illuminated intact chloroplast in the presence of membrane-modifying agents. Plant Sci Lett 7:101–107
Vredenberg WJ, Bulychev AA (2003) Photoelectric effects on chlorophyll fluorescence of photosystem II in vivo. Kinetics in the absence and presence of valinomycin. Bioelectrochemistry 60:87–95
Vredenberg WJ, Bulychev AA (2010) Photoelectrochemical control of the balance between cyclic- and linear electron transport in photosystem I. Algorithm for P700+ induction kinetics. Biochim Biophys Acta 1797:1521–1532
Vredenberg W, Durchan M, Prasil O (2007) On the chlorophyll a fluorescence yield in chloroplasts upon excitation with twin turnover flashes (TTF) and high frequency flash trains. Photosynth Res 93:183–192
Vredenberg W, Durchan M, Prášil O (2009) Photochemical and photoelectrochemical quenching of chlorophyll fluorescence in photosystem II. Biochim Biophys Acta 1787:1468–1478
Wraight CA, Crofts AR (1971) Delayed fluorescence and the high-energy state of chloroplasts. Eur J Biochem 19:386–397
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bulychev, A.A., Osipov, V.A., Matorin, D.N. et al. Effects of far-red light on fluorescence induction in infiltrated pea leaves under diminished ΔpH and Δφ components of the proton motive force. J Bioenerg Biomembr 45, 37–45 (2013). https://doi.org/10.1007/s10863-012-9476-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10863-012-9476-6