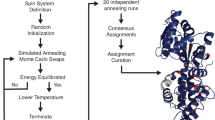
Abstract
An NMR investigation of proteins with known X-ray structures is of interest in a number of endeavors. Performing these studies through nuclear magnetic resonance (NMR) requires the costly step of resonance assignment. The prevalent assignment strategy does not make use of existing structural information and requires uniform isotope labeling. Here we present a rapid and cost-effective method of assigning NMR data to an existing structure—either an X-ray or computationally modeled structure. The presented method, Exhaustively Permuted Assignment of RDCs (EPAR), utilizes unassigned residual dipolar coupling (RDC) data that can easily be obtained by NMR spectroscopy. The algorithm uses only the backbone N–H RDCs from multiple alignment media along with the amino acid type of the RDCs. It is inspired by previous work from Zweckstetter and provides several extensions. We present results on 13 synthetic and experimental datasets from 8 different structures, including two homodimers. Using just two alignment media, EPAR achieves an average assignment accuracy greater than 80%. With three media, the average accuracy is higher than 94%. The algorithm also outputs a prediction of the assignment accuracy, which has a correlation of 0.77 to the true accuracy. This prediction score can be used to establish the needed confidence in assignment accuracy.




Similar content being viewed by others
References
Al-Hashimi HM, Bolon PJ, Prestegard JH (2000) Molecular symmetry as an aid to geometry determination in ligand protein complexes. J Magn Reson 142:153–158
Bansal S, Miao X, Adams MWW et al (2008) Rapid classification of protein structure models using unassigned backbone RDCs and probability density profile analysis (PDPA). J Magn Reson 192:60–68
Bax A (2003) Weak alignment offers new NMR opportunities to study protein structure and dynamics. Protein Sci 12:1–16
Bax A, Grishaev A (2005) Weak alignment NMR: a hawk-eyed view of biomolecular structure. Curr Opin Struct Biol 15:563–570
Bax A, Kontaxis G, Tjandra N (2001) Dipolar couplings in macromolecular structure determination. Methods Enzymol 339:127–174
Berman HM, Westbrook J, Feng Z et al (2000) The protein data bank. Nucleic Acids Res 28:235–242
Chen C, Cheng C, Chen Y et al (2006) Preparation of amino-acid-type selective isotope labeling of protein expressed in Pichia pastoris. Proteins 62:279–287
Clore GM, Gronenborn AM, Bax A (1998) A robust method for determining the magnitude of the fully asymmetric alignment tensor of oriented macromolecules in the absence of structural information. J Magn Reson 133:216–221
Cornilescu G, Marquardt JL, Ottiger M et al (1998) Validation of protein structure from anisotropic carbonyl chemical shifts in a dilute liquid crystalline phase. Journal of American Chemical Society 120:6836–6837
Greshenfeld NA (1998) The nature of mathematical modeling. Cambridge University Press, Cambridge
Gronenborn AM, Clore GM (1996) Rapid screening for structural integrity of expressed proteins by heteronuclear NMR spectroscopy. Protein Sci 5:174–177
Grzesiek S, Bax A (1993) Amino acid type determination in the sequential assignment procedure of uniformly 13C/15 N-enriched proteins. J Biomol NMR 3:185–204
Hus J, Prompers J, Bruschweiler R (2002) Assignment strategy for proteins with known structure. J Magn Reson 157:119–123
Jones EY, Davis SJ, Williams AF et al (1992) Crystal structure at 2.8Å resolution of a soluble form of the cell adhesion molecule CD2. Nature 360:232–239
Jung Y, Zweckstetter M (2004) Backbone assignment of proteins with known structure using residual dipolar couplings. J Biomol NMR V30:25–35
Kuhn HW (1955) The Hungarian method for the assignment problem. Naval Res Logist Q 2:83–97
Langmead CJ, Donald BR (2004) An expectation/maximization nuclear vector replacement algorithm for automated NMR resonance assignments. J Biomol NMR 29:111–138
Langmead CJ, Yan A, Lilien R et al (2004) A polynomial-time nuclear vector replacement algorithm for automated NMR resonance assignments. J Comput Biol 11:277–298
Leopold M, Urbauer J, Wand A (1994) Resonance assignment strategies for the analysis of NMR spectra of proteins. Mol Biotechnol 2:61–93
Losonczi JA, Andrec M, Fischer MWF, Prestegard JH (1999) Order matrix analysis of residual dipolar couplings using singular value decomposition. J Magn Reson 138:334–342
Miao X, Mukhopadhyay R, Valafar H (2008) Estimation of relative order tensors, and reconstruction of vectors in space using unassigned RDC data and its application. J Magn Reson 194:202–211
Mukhopadhyay R, Shealy P, Valafar H (2008) Protein fold family recognition from unassigned residual dipolar coupling data, 633–638
Murray AJ, Head JG, Barker JJ et al (1998) Engineering an intertwined form of CD2 for stability and assembly. Nat Struct Biol 5:778–782
Ou HD, Lai HC, Serber Z et al (2001) Efficient identification of amino acid types for fast protein backbone assignments. J Biomol NMR 21:269–273
Park SH, Son WS, Mukhopadhyay R et al (2009) Phage-induced alignment of membrane proteins enables the measurement and structural analysis of residual dipolar couplings with dipolar waves and lambda-maps. J Am Chem Soc 131:14140–14141
Pons JL, Delsuc MA (1999) RESCUE: an artificial neural network tool for the NMR spectral assignment of proteins. J Biomol NMR 15:15–26
Press W, Teukolsky SA, Vetterling WT, Flannery BP (2002) Numerical recipes in C: the art of scientific computing. Cambridge University Press, Cambridge
Prestegard JH, al-Hashimi HM, Tolman JR (2000) NMR structures of biomolecules using field oriented media and residual dipolar couplings. Q Rev Biophys 33:371–424
Saupe A, Englert G (1963) High-resolution nuclear magnetic resonance spectra of orientated molecules. Phys Rev Lett 11:462–464
Schwieters CD, Kuszewski JJ, Tjandra N et al (2003) The Xplor-NIH NMR molecular structure determination package. J Magn Reson 160:65–73
Spera S, Bax A (1991) Empirical correlation between protein backbone conformation and C.alpha. and C.beta. 13C nuclear magnetic resonance chemical shifts. J Am Chem Soc 113:5490–5492
Tian F, Valafar H, Prestegard JH (2001) A dipolar coupling based strategy for simultaneous resonance assignment and structure determination of protein backbones. J Am Chem Soc 123:11791–11796
Tolman JR, Flanagan JM, Kennedy MA et al (1995) Nuclear magnetic dipole interactions in field-oriented proteins—information for structure determination in solution. Proc Natl Acad Sci USA 92:9279–9283
Tong K, Yamamoto M, Tanaka T (2008) A simple method for amino acid selective isotope labeling of recombinant proteins in E. coli. J Biomol NMR 42:59–67
Ulmer TS, Ramirez BE, Delaglio F et al (2003) Evaluation of backbone proton positions and dynamics in a small protein by liquid crystal NMR spectroscopy. J Am Chem Soc 125:9179–9191
Ulrich EL, Akutsu H, Doreleijers JF et al (2008) BioMagResBank. Nucleic Acids Res 36:D402–D408
Valafar H, Prestegard J (2004) REDCAT: a residual dipolar coupling analysis tool. J Magn Reson 167:228–241
Varner S, Vold R, Hoatson G (1996) An efficient method for calculating powder patterns. J Magn Reson 123:72–80
Warren JJ, Moore PB (2001) A maximum likelihood method for determining D(a)(PQ) and R for sets of dipolar coupling data. J Magn Reson 149:271–275
Whittaker J (2007) Selective isotopic labeling of recombinant proteins using amino acid auxotroph strains. Methods Mol Biol 389:175–187
Zweckstetter M (2003) Determination of molecular alignment tensors without backbone resonance assignment: aid to rapid analysis of protein–protein interactions. J Biomol NMR 27:41–56
Zweckstetter M, Bax A (2000) Prediction of sterically induced alignment in a dilute liquid crystalline phase: aid to protein structure determination by NMR. J Am Chem Soc 122:3791–3792
Acknowledgments
This work has been funded by NSF Grant number MCB-0644195. The authors are grateful to the Rothberg fellowship at USC for support to PGS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shealy, P., Liu, Y., Simin, M. et al. Backbone resonance assignment and order tensor estimation using residual dipolar couplings. J Biomol NMR 50, 357–369 (2011). https://doi.org/10.1007/s10858-011-9521-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10858-011-9521-5