Abstract
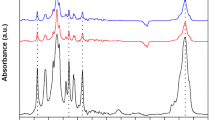
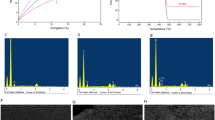
Polypropylene mesh materials have been utilized in hernia surgery for over 40 years. However, they are prone to degradation due to the body’s aggressive foreign body reaction, which may cause pain or complications, forcing mesh removal from the patient. To mitigate these complications, gold nanomaterials were attached to polypropylene mesh in order to improve cellular response. Pristine samples of polypropylene mesh were exposed to hydrogen peroxide/cobalt chloride solutions to induce formation of surface carboxyl functional groups. Gold nanoparticles were covalently linked to the mesh. Scanning electron microscopy confirmed the presence of gold nanoparticles. Differential scanning calorimetry and mechanical testing confirmed that the polypropylene did not undergo any significantly detrimental changes in physicochemical properties. A WST-1 cell culture study showed an increase in cellularity on the gold nanoparticle–polypropylene mesh as compared to pristine mesh. This study showed that biocompatibility of polypropylene mesh may be improved via the conjugation of gold nanoparticles.





Similar content being viewed by others
References
Brown CN, Finch JG. Which mesh for hernia repair? Ann R Coll Surg Engl. 2010;92(4):272–8.
Bringman S, Conze J, Cuccurullo D, Deprest J, Junge K, Klosterhalfen B, Parra-Davila E, Ramshaw B, Schumpelick V. Hernia repair: the search for ideal meshes. Hernia. 2010;14(1):81–7.
Yang J, Papandria D, Rhee D, Perry H, Abdullah F. Low-cost mesh for inguinal hernia repair in resource-limited settings. Hernia. 2011; In press.
Voskerician G, Jin J, White M, Williams C, Rosen M. Effect of biomaterial design criteria on the performance of surgical meshes for abdominal hernia repair: a pre-clinical evaluation in a chronic rat model. J Mater Sci Mater Med. 2010;21(6):1989–95.
Nelson EC, Vidovszky TJ. Composite mesh migration into the sigmoid colon following ventral hernia repair. Hernia. 2011;15(1):47–52.
Cozad MJ, Ramshaw BR, Grant DN, Bachman SL, Grant DA, Grant SA. Materials characterization of explanted polypropylene, polyethylene terephthalate, and expanded polytetrafluoroethylene composites: spectral and thermal analysis. J Biomed Mater Res B. 2010;49B:455–62.
Sutherland K, Mahoney JR, Coury AJ, Eaton JW. Degradation of biomaterials by phagocyte-derived oxidants. J Clin Invest. 1993;92:2360–7.
Costello CR, Bachman SL, Ramshaw BR, Grant SA. Materials characterization of explanted heavyweight polypropylene hernia meshes. J Biomed Mater Res B. 2007;83B:44–9.
Zinther NB, Wara P, Friis-Andersen H. Shrinkage of intraperitoneal onlay mesh in sheep: coated polyester mesh versus covered polypropylene mesh. Hernia. 2010;14(6):611–5.
Beldi G, Wagner M, Bruegger LE, Kurmann A, Candinas D. Mesh shrinkage and pain in laparoscopic ventral hernia repair: a randomized clinical trial comparing suture versus tack mesh fixation. Surg Endosc. 2010;24(6):1451–5.
Khan RN, Kindal V, Bansal VK, Misra MC, Kumar S. Does mesh shrinkage in any way depend upon the method of mesh fixation in laparoscopic incisional hernia repair? Surgical Endoscopy. 2010. doi:10.1007/s00464-010-1363-9.
Downey DM, Dubose JJ, Ritter TA, Dolan JP. Validation of a radiographic model for the assessment of mesh migration. J Surg Res. 2011;166:109–13.
Bhattacharyya AR, Sreekumar TV, Liu T, Kumar S, Ericson LM, Hauge RH, Smalley RE. Crystallization and orientation studies in polypropylene/single wall carbon nanotube composite. Polymers. 2003;44(8):2373–7.
Bohm, G, Ushakova Y, Alizai H, Braunschweig T, Lente C, Heffels K, Groll J, Neumann U, Junge D. Biocompatibility of PLGA/sP(EO-stat-PO)-coated mesh surfaces under constant shearing stress. Eur Surg Res. 2011;47(3):118–129.
Hsu S, Tang C, Tseng H. Biocompatibility of poly(ether)urethane-gold nanocomposites. J Biomed Mater Res A. 2006;79:759–70.
Chou C, Hsu S, Wang P. Biostability and biocompatibility of poly(ether)urethane containing gold or silver nanoparticles in a porcine model. J Biomed Mater Res A. 2007;84:785–94.
Kalayci O, Comert F, Hazer B, Atalay T, Cavicchi K, Cakmak M. Synthesis, characterization, and antibacterial activity of metal nanoparticles embedded into amphiphilic comb-type graft copolymers. Polym Bull. 2010;65:215–26.
Christenson EM, Anseth KS, van den Beucken LJ, Chan CK, Ercan B, Jansen JA, Laurencin CT, Li WJ, Murugan R, Nair LS. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25:11–22.
Balasundaram G, Webster TJ. A perspective on nanophase materials for orthopedic implant applications. J Mater Chem. 2006;16:3737–45.
Dillow AK, Lowman AM. Biomimetic materials and design. New York: Marcel Dekker Inc.; 2002.
Kay S, Thapa A, Haberstroh KM, Webster TJ. Nanostructured polymer/nanophase ceramic composites enhance osteoblast and chondrocyte adhesion. Tissue Eng. 2002;8:753–61.
Sniakecki NJ, Desai RA, Ruiz SA, Chen CS. Nanotechnology for cell-substrate interactions. Ann Biomed Eng. 2006;34(1):59–74.
Teixeira AI, Nealey PF, Murphy CJ. Responses of human keratocytes to micro-nanostructured substrates. J Biomed Mater Res. 2004;71A:3153–64.
Yim EK, Reano RM, Pang SW, Yee AF, Chen CS, Leong KW. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials. 2005;26:5405–13.
Curtis AS, Wilkinson CD, Crossan J, Broadley C, Darmani H, Johal KK, Jorgensen H, Monaghan W. An in vivo microfabricated scaffold for tendon repair. Eur Cell Mater. 2005;9:50–7.
Foley JD, Grunwald E, Nealey PF, Murphy CJ. Cooperative modulation of neuritogenesis by PC12 cells by topography and nerve growth factor. Biomaterials. 2005;26:3639–44.
Dalby MJ, Yarwood SJ, Riehle MO, Johnstone HJ, Affrossman S, Curtis AS. Increasing fibroblast response to materials using nanotopogrpahy: Morphological and genetic measurements of cell response to 13-nm-high- polymer demixed islands. Exp Cell Res. 2002;276:2002.
Christenson EM, Anderson JM, Hiltner A. Oxidative mechanisms of poly(carbonate urethane) and poly(ether urethane) biodegradation: In vivo and in vitro correlations. J Biomed Mat Res A. 2004;70(2):245–55.
Deeken CR, Fox DB, Bachman SL, Ramshaw BJ, Grant SA. Characterization of bionanocomposite scaffolds comprised of amine-functionalized gold nanoparticles and silicon carbide nanowires crosslinked to an acellular porcine tendon. J Biomed Mater Res B. 2011;99(1):142–9.
Deeken CR, Fox DB, Bachman SL, Ramshaw BJ, Grant SA. Assessment of the biocompatibility of two novel, bionanocomposite scaffolds in a rodent model. J Biomed Mater Res B. 2011;96B(2):351–9.
Hermanson G. Preparation of colloidal-gold-labeled proteins, bioconjugate techniques. New York: Academic Press; 1996.
Badylak SF, Lantz GC, Coffey A, Geddes LA. Small intestinal submucosa as a large diameter vascular graft in the dog. J Surg Res. 1989;47:74–80.
Costello CR, Bachman SL, Grant SA, Cleveland DS, Loy TS, Ramshaw BR. Characterization of heavyweight and lightweight polypropylene prosthetic mesh explants from a single patient. Surg Innov. 2007;14(3):168–76.
Cobb WS, Burns JM, Kercher KW, Matthews BD, Norton HJ, Heniford BT. Normal intraabdominal pressure in healthy adults. J Surg Res. 2005;129:231–5.
Emans P, Schreinemacher M, Gijbels M, Beets G, Greve J-W, Koole L, Bouvy N. Polypropylene meshes to prevent abdominal herniation can stable coatings prevent adhesions in the long term? Ann Biomed Eng. 2009;37(2):410–8.
Acknowledgments
The authors would like to acknowledge funding from the Missouri F21C Food for the 21st Century.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grant, D.N., Benson, J., Cozad, M.J. et al. Conjugation of gold nanoparticles to polypropylene mesh for enhanced biocompatibility. J Mater Sci: Mater Med 22, 2803–2812 (2011). https://doi.org/10.1007/s10856-011-4449-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10856-011-4449-6