Abstract
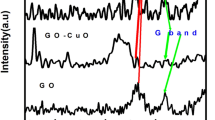
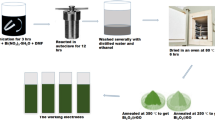
In the present work, bismuth iron oxide–graphene composite has been explored as electrode material for electrochemical supercapacitor application. Bismuth iron oxide (BFO) nanoparticles, synthesized by sol–gel process, are mixed with the graphene sheets in a solution. The electrodes are prepared by coating the resulted slurry on stainless steel (SS) substrate, by drop casting process. The morphology and structure of the BFO–graphene composite are characterized by XRD, FIB-SEM, HRTEM and Raman spectroscopy, which show that the nanoparticles with diameter 100–200 nm are randomly distributed on and around the graphene sheets. The composite electrode exhibits significantly enhanced capacitance as compared to BFO. In this structure, the electrons generated by the surface based Faradaic reactions from the BFO nanoparticles can be transported by the graphene nanosheets toward the current collector. The electrochemical characteristic of the electrodes is investigated through cyclic voltammetry and charging/discharging process. The specific capacitance of the electrode measured at 5–100 mV/s was found to be 17–4 mF/cm2 which is comparable to the most commonly used metal oxide based electrode materials. It shows better cycling stability with 95% retention of capacitance after 2000 cycles.







Similar content being viewed by others
References
R. Kotz, M. Carlen, Principles and applications of electrochemical capacitors. Electrochim. Acta 45, 2483–2498 (2000)
S.F. Tie, C. Wei, A review of energy sources and energy management system in electric vehicles. Renew. Sustain. Energy Rev. 20, 82–102 (2013)
A. Kuperman, I. Aharon, Battery—ultracapacitor hybrids for pulsed current loads: a review. Renew. Sustain. Energy Rev. 15, 981–992 (2011)
J.R. Miller, A.F. Burke, Electrochemical capacitors: challenges and opportunities for real-world applications. Electrochem. Soc. Interface 17, 53–57 (2008)
D.P. Dubal, G. Kim, Y. Kim, R. Holze, C.D. Lokhande, W.B. Kim, Supercapacitors based on flexible substrates: an overview. Energy Technol. 2, 325–341 (2014)
T. Lé, P. Gentile, G. Bidan, D. Aradilla, New electrolyte mixture of propylene carbonate and butyltrimethylammonium bis(trifluoromethylsulfonyl)imide (N1114 TFSI) for high performance silicon nanowire (SiNW)-based supercapacitor applications. Electrochim. Acta 254, 368–374 (2017)
M.K. Hota, Q. Jiang, Y. Mashraei, K.N. Salama, H.N. Alshareef, Fractal electrochemical microsupercapacitors. Adv. Electron. Mater. 3, 1700185 (2017)
K. Zhang, L.L. Zhang, X.S. Zhao, J. Wu, Graphene/polyaniline nanofiber composites as supercapacitor electrodes. Chem. Mater. 22, 1392–1401 (2010)
L. Gurusamy, S. Anandan, J.J. Wu, Synthesis of reduced graphene oxide supported flower-like bismuth subcarbonates microsphere (Bi2O2CO3-RGO) for supercapacitor. Appl. Electrochim. Acta 244, 209–221 (2017)
C.D. Lokhande, D.P. Dubal, O. Joo, Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 11, 255–270 (2017)
Q.J. Le, T. Wang, D.N.H. Tran, F. Dong, Y.X. Zhang, D. Losic, Morphology-controlled MnO2 modified silicon diatoms for high-performance asymmetric supercapacitors. J. Mater. Chem. A 5, 10856–10865 (2017)
A. Soam, N. Arya, A. Singh, R. Dusane, Fabrication of silicon nanowires based on-chip micro-supercapacitor. Chem. Phys. Lett. 678, 45–50 (2017)
E. Frackowiak, K. Metenier, V. Bertagna, F. Beguin, Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 77, 2421–2423 (2010)
A.G. Pandolfo, A.F. Hollenkamp, Carbon properties and their role in supercapacitors. J. Power Sources 157, 11–27 (2006)
P. Chen, G. Shen, S. Sukcharoenchoke, C. Zhou, Flexible and transparent supercapacitor based on In 2O3 nanowire/carbon nanotube heterogeneous films. Appl. Phys. Lett. 94, 043113 (2009)
A.L.M. Reddy, S. Ramaprabhu, Nanocrystalline metal oxides dispersed multiwalled carbon nanotubes as supercapacitor electrodes. J. Phys. Chem. C 111, 7727–7734 (2007)
V. Khomenko, E. Frackowiak, F. Be, Performance of manganese oxide CNTs composites as electrode materials for electrochemical capacitors. J. Electrochem. Soc. 152, 229–235 (2005)
Y. Zhang, H. Li, L. Pan, T. Lu, Z. Sun, Capacitive behavior of graphene—ZnO composite film for supercapacitors. J. Electroanal. Chem. 634, 68–71 (2009)
A. Soam, P. Kavle, A. Kumbhar, R.O. Dusane, Performance enhancement of micro-supercapacitor by coating of graphene on silicon nanowires at room temperature. Curr. Appl. Phys. 17, 68–71 (2017)
H. Wu, J. Zhou, L. Liang, L. Li, X. Zhu, Fabrication, characterization, properties, and applications of low-dimensional BiFeO3 nanostructures. J. Nanomater. 2014, 471485 (2014)
S. Nayak, C. Mahender, S. Ankur, J. Nanda, Structural and optical studies of BiFeO3@SiO2 core/shell nanoparticles. Mater. Res. Express 4, 105029 (2017)
L. Di, H. Yang, T. Xian, X. Chen, Enhanced photocatalytic activity of NaBH4 reduced BiFeO3 nanoparticles for rhodamine B decolorization. Materials 10, 1118 (2017)
P. Banerjee, A.F. Jr, Influence of Y and Co co-doping in the multiferroic behaviors of BiFeO3 ceramics. J. Mater. Sci. 28, 8562–8568 (2017)
A. Sarkar, A.K. Singh, D. Sarkar, G.G. Khan, K. Mandal, Three-dimensional nanoarchitecture of BiFeO3 anchored TiO2 nanotube arrays for electrochemical energy storage and solar energy conversion. ACS Sustain. Chem. Eng. 3, 2254–2263 (2015)
C. Lokhande, T. Gujar, R.S. Mane, S.H. Han, Electrochemical supercapacitor application of pervoskite thin films. Electrochem. Commun. 9, 1805–1809 (2007)
V.V. Jadhav, M.K. Zate, S. Liu, M. Naushad, R.S. Mane, K.N. Hui, S.H. Han, Mixed-phase bismuth ferrite nanoflake electrodes for supercapacitor application. Appl. Nanosci. 6, 511–519 (2016)
M.P. Tereza, A.K. Thapa, A. Sherehiy, J.B. Jasinski, J.S. Jangam, Incommensurate graphene foam as a high capacity lithium intercalation anode. Sci. Rep. 7, 39944 (2017)
P. Priyadharsini, A. Pradeep, B. Sathyamoorthy, G. Chandrasekaran, Enhanced multiferroic properties in La and Ce. J. Phys. Chem. Solids 75, 797–802 (2014)
A. Sun, H. Chen, C. Song, F. Jiang, X. Wang, Y. Fu, Magnetic Bi25FeO40-graphene catalyst and its high visible-light photocatalytic performance. RSC Adv. 3, 4332–4340 (2013)
A. Kaniyoor, S. Ramaprabhu, A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2, 032183 (2012)
H. Pan, J. Li, Y.P. Feng, Carbon nanotubes for supercapacitor. Nanoscale Res. Lett. 5(3), 654–668 (2010)
B. Senthilkumar, R.K. Selvan, L. Vasylechko, M. Minakshi, Synthesis, crystal structure and pseudocapacitor electrode properties of γ-Bi2MoO6 nanoplates. Solid State Sci. 35, 18–27 (2014)
W. Liu, C. Lu, X. Wang, R.Y. Tay, B.K. Tay, High-performance microsupercapacitors based on two-dimensional graphene/manganese dioxide/silver nanowire ternary hybrid film. ACS Nano 9, 1528–1542 (2015)
B. Sarma, A.L. Jurovitzki, Y.R. Smith, S.K. Mohanty, M. Misra, Redox-induced enhancement in interfacial capacitance of the titania nanotube/bismuth oxide composite electrode. ACS Appl. Mater. Interfaces 5, 1688–1697 (2013)
W.G. Pell, B.E. Conway, Analysis of power limitations at porous supercapacitor electrodes under cyclic voltammetry modulation and dc charge. J. Power Sources 96, 57–67 (2001)
W.G. Pell, B.E. Conway, N. Marincic, Analysis of non-uniform charge/discharge and rate effects in porous carbon capacitors containing sub-optimal electrolyte concentrations. J. Electroanal. Chem. 491, 9–21 (2000)
R.D. Levie, On porous electrodes in electrolyte solutions: I. capacitance effects. Electrochim. Acta 8, 751–780 (1963)
Acknowledgements
I would like to thank Prof. V. S. Raja, Dept. of ME & MS, IIT Bombay for providing the electrochemical characterization facility. FIST facility (Dual beam FIB, Carl Zeiss Microscopy) in ME & MS department was also used for this work. I also acknowledge SAIF, IIT Bombay, Mumbai for HRTEM characterization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nayak, S., Soam, A., Nanda, J. et al. Sol–gel synthesized BiFeO3–Graphene nanocomposite as efficient electrode for supercapacitor application. J Mater Sci: Mater Electron 29, 9361–9368 (2018). https://doi.org/10.1007/s10854-018-8967-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-8967-6