Abstract
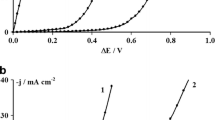
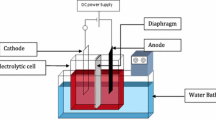
Lead (Pb)–cobalt (Co) films have been studied by several researchers regarding their electrocatalytic activity when used as anodes in oxygen evolution reactions. Although several authors have suggested that the electrodeposition technique is the best alternative to Pb–Co film deposition, the process is complex. In many cases, the use of complexing agents facilitates the electrodeposition of multi-element coatings; but this performance has not been reported in the literature for Pb–Co electrodeposition. In this work, the effect of three different complexing agents (tartaric acid, sodium citrate, and ascorbic acid) on Pb–Co electrodeposition was studied. Speciation diagrams and polarization curves have been used to determine the reduction potentials of Pb2+ and Co2+ ions in the different systems studied. Speciation diagram predictions were incapable of revealing all species present in solution and for an accurate prediction of the actual electrochemical functioning of the system; the information given by the speciation diagrams and polarization curves must be considered together. Additionally, electrodeposition of Pb–Co films was achieved without complexing agent and using the three complexing agents. Morphological and chemical properties of these coatings are shown and discussed in terms of the electrochemical functioning of each system. Finally, the electrocatalytic ability of the Pb–Co films was investigated by means of polarization curves.









Similar content being viewed by others
References
Nikoloski A, Nicol M (2010) Addition of cobalt to lead anodes used for oxygen evolution—a literature review. Miner Process Extr Metall Rev 31:30–57
Neuróhr K, Dégi J, Pogáni L, Bakony I, Ungvári D, Vad K, Hakl J, Révész A, Péter L (2012) Composition, morphology and electrical transport properties of Co–Pb electrodeposits. J Alloys Compd 545:111–112
Barmi M, Nikoloski A (2012) Electrodeposition of lead–cobalt composite coatings electrocatalytic for oxygen evolution and the properties of composite coated anodes for copper electrowinning. Hydrometallurgy 129–130:59–66
Rashkov S, Dobrev T, Noncheva Z, Stefanov Y, Rashkova B, Petrova M (1999) Lead–cobalt anodes for electrowinning of zinc from sulphate electrolytes. Hydrometallurgy 52:223–230
Cheng K, Chiang W (2011) Effect of [Cu]/[Cu + In] ratio in the solution bath on the growth and physical properties of CuInS2 film using one-step electrodeposition. J Electroanal Chem 661:57–65
Haitao Y, Buming C, Jianhua L, Zhonchen G, Yongchun Z, Ruidong X (2014) Preparation and properties of Al/Pb-Ag-Co composite anode material for zinc electrowinning. Rare Met Mater Eng 43:2889–2892
Yao C, Ma H, Zhang X, Meng L, Zhao L, Tai L, Wang Y, Gong Q, Tong Y (2011) Electrochemical preparation and magnetic properties of submicron CoxPb1−x dendrites. J Solid State Electrochem 15:1193–1199
Frank A, Sumodjo P (2014) Electrodeposition of cobalt from citrate containing baths. Electrochim Acta 132:75–82
Serruys Y, Sakout T, Gorse D (1993) Anodic oxidation of titanium in 1M H2SO4, studied by Rutherford backscattering. Surf Sci 282:279
Collinson M, Higgins D, Kommidi R, Campbel D (2008) Electrodesposited silicate films: importance of supporting electrolyte. Anal Chem 80:651–656
Li F, Wang W (2009) Electrodeposition of BixSb2-xTex thermoelectric thin films from nitric acid and hydrochloric acidic systems. Appl Surf Sci 255:4225–4231
Jayakumar M, Venkatesan K, Sudha R, Srinivasan T, Vasudeva P (2011) Electrodeposition of ruthenium, rhodium and palladium from nitric acid and ionic liquid media: recovery and Surface morphology of deposits. Mater Chem Phys 128:141–150
Krotz R, Evangelou B, Wagner G (1989) Relationships between cadmium, zinc, Cd-Peptide, and organic acid in tobacco suspension cells. Plant Physiol 91:780–787
Dean JA (1999) Lange’s handbook of chemistry, 15th edn. McGraw-Hill Inc, New York
Wong K, Lee C, Low K, Haron M (2003) Removal of Cu and Pb by tartaric acid modifiedrice husk from aqueous solutions. Chemosphere 50:23–28
Ishizaki T, Ohomoto T, Sakamoto Y, Fuwa A (2004) Effect of pH on the electrodeposition of ZnTe film from a citric acid solution. Mater Trans 45:277–280
Martell A, Smith R (1989) Critical stability constants second supplement. Springer Science + Business Media, Berlin
Branica G, Metikos M, Omanovic D (2006) Voltammetric determination of stability constants of lead complexes with vitamin C. Croatia Chem Acta 79:77–83
Sachan S, Singh P, Kumar P, Nigan V, Singh K (2011) Electrophoretic studies of biologically important mixed metal-ascorbic acid-nitriloacetate complexes. Croat Chem Acta 84:461–464
Aghassi A, Jafarian M, Danaee I, Gobal F, Mahjani M (2011) AC impedance and cyclic voltammetry studies on PbS semiconducting film prepared by electrodeposition. J Electroanal Chem 661:265–269
Sharon M, Ramaian K, Kumarm M, Neumann M, Levy C (1997) Electrodeposition of lead sulphide in acidic medium. J Electroanal Chem 436:49–52
Liang D, Liu Z, Hilty R, Zangari G (2012) Electrodeposition of Ag–Ni films from thiourea complexing solutions. Electrochim Acta 82:82–89
Oury A, Kirchev A, Bultel Y, Chainet E (2012) PbO2/Pb2+ cycling in methanesulfonic acid and mechanisms associated for soluble lead-acid flow battery applications. Electrochim Acta 71:140–149
Arami H, Mazloumi M, Khalifehzadeh R, Sadrnezhaad S (2008) Surfactant free hydrothermal formation of Pb3O4 nanorods. J Alloys Compd 466:323–325
Jeffrey M, Choo W, Breuer P (2000) The effect of additives and impurities on the cobalt electrowinning process. Miner Eng 13:1231–1241
García E, Santos J, Pereira E, Freitas M (2008) Electrodeposition of cobalt from spent Li-ion battery cathodes by the electrochemistry quartz crystal microbalance technique. J Power Sources 185:549–553
Matsushima J, Trivinho F, Pereira E (2006) Investigation of cobalt deposition using the electrochemical quartz crystal microbalance. Electrochim Acta 51:1960–1966
Freitas M, García E (2007) Electrochemical recycling of cobalt from cathodes of spent lithium-ion batteries. J Power Sources 171:953–959
Pandey P, Sahu S, Chandra S (1996) Handbook of semiconductor electrodeposition. Taylor and Francis, New York
Rodríguez C, Sandoval M, Cabello G, Flores M, Fernández H, Carrasco C (2014) Characterization of ZnS thin films synthesized through a non-toxic precursors chemical bath. Mater Res Bull 60:313–321
Davies M (1992) Reactions of L-ascorbic acid with transition metals complexes. Polyhedron 11:285–321
Khan M, Sarwar A (2001) The influence of transition metal ions on the kinetics of ascorbic acid oxidation by methylene blue in strongly acidic media. Turk J Chem 25:433–440
Lu L, Wang Y, Li X (2012) Influence of processing parameters on the preparation of CuInS2 thin film by one-step electrodeposition as the solar cell absorber. Surf Coat Technol 212:55–60
Musiani M, Furlanetto F, Bertoncello R (1999) Electrodeposited PbO2 + RuO2: a composite anode for oxygen evolution from sulphuric acid solution. J Electroanal Chem 465:160–167
Kato Z, Kashima R, Tatsumi K, Fukuyama S, Izumiya K, Kumagai M, Hashimoto K (2016) The influence of coating solution and calcination condition on thedurability of Ir1-xSnxO2/Ti anodes for oxygen evolution. Appl Surf Sci. doi:10.1016/j.apsusc.2016.03.071
Acknowledgements
Authors acknowledge the financial support by CONICYT through Regular FONDECYT project 1150270 and the invaluable motivation received from of Dr. Petr Vanysek.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
All the authors of the manuscript declare no conflicts of interest that could potentially influence or bias the submitted document.
Rights and permissions
About this article
Cite this article
Rodríguez, C.A., Tobosque, P., Maril, M. et al. Effect of different complexing agents on Pb–Co thin-film electrodeposition. J Mater Sci 52, 3388–3401 (2017). https://doi.org/10.1007/s10853-016-0627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0627-8