Abstract
A series of 4,9-dihydro-s-indaceno[1,2-b:5,6-b′]dithiophene-based conjugated polymers with 3,4-bis(dodecyloxy)thiophene, 3,3-bis((dodecyloxy)methyl)-3,4-dihydro-2H-thieno[3,4-b] [1, 4] dioxepine and 3,4-ethylenedioxythiophene as co-monomers were synthesized via Stille coupling reaction in more than 50 % yields. These polymers possess relatively high number-average molecular weights of 14,900–21,000 g mol−1 and exhibit good solubility in common organic solvents. The physical and electrochemical properties of these polymers were studied, and the polymers showed optical bandgap between 2.04 and 2.08 eV. Electrochromic devices showed a rare and reversible colour change between dark red at neutral state and black at oxidized state with optical switching contrast ratios of up to 50 % at λ max and 90 % at 1500 nm in the visible and NIR regions, respectively.
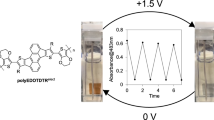
Graphical abstract
4,9-dihydro-s-indaceno[1,2-b:5,6-b’]dithiophene-based conjugated polymers showing rare and reversible colour change between dark red at neutral state and black at oxidized state were synthesized via Stille coupling. These polymers possess high optical contrast in both the visible and NIR regions.








Similar content being viewed by others
References
Cheng Y-J, Yang S-H, Hsu C-S (2009) Synthesis of conjugated polymers for organic solar cell applications. Chem Rev 109(11):5868–5923
Duan CH, Huang F, Cao Y (2012) Recent development of push-pull conjugated polymers for bulk-heterojunction photovoltaics: rational design and fine tailoring of molecular structures. J Mater Chem 22(21):10416–10434
Guenes S, Neugebauer H, Sariciftci NS (2007) Conjugated polymer-based organic solar cells. Chem Rev 107(4):1324–1338
Boudreault P-LT, Najari A, Leclerc M (2011) Processable low-bandgap polymers for photovoltaic applications. Chem Mater 23(3):456–469
Kulkarni AP, Tonzola CJ, Babel A, Jenekhe SA (2004) Electron transport materials for organic light-emitting diodes. Chem Mater 16(23):4556–4573
Bernius MT, Inbasekaran M, O’Brien J, Wu WS (2000) Progress with light-emitting polymers. Adv Mater 12(23):1737–1750
Kamtekar KT, Monkman AP, Bryce MR (2010) Recent advances in white organic light-emitting materials and devices (WOLEDs). Adv Mater 22(5):572–582
Lu K, Liu Y (2010) Polythiophenes: important conjugated semiconducting polymers for organic field-effect transistors. Curr Org Chem 14(18):2017–2033
Wang CL, Dong HL, Hu WP, Liu YQ, Zhu DB (2012) Semiconducting pi-conjugated systems in field-effect transistors: a material odyssey of organic electronics. Chem Rev 112(4):2208–2267
Mei C-Y, Liang L, Zhao F-G, Wang J-T, Yu L-F, Li Y-X, Li W-S (2013) A family of donor-acceptor photovoltaic polymers with fused 4,7-dithienyl-2,1,3-benzothiadiazole units: effect of structural fusion and side chains. Macromolecules 46(19):7920–7931
Biniek L, Schroeder BC, Nielsen CB, McCulloch I (2012) Recent advances in high mobility donor-acceptor semiconducting polymers. J Mater Chem 22(30):14803–14813
Beaujuge PM, Reynolds JR (2010) Color control in π-conjugated organic polymers for use in electrochromic devices. Chem Rev 110(1):268–320
Balan A, Baran D, Toppare L (2011) Benzotriazole containing conjugated polymers for multipurpose organic electronic applications. Polym Chem 2(5):1029–1043
Sonmez G (2005) Polymeric electrochromics. Chem Commun 42:5251–5259
Thakur VK, Ding G, Ma J, Lee PS, Lu X (2012) Hybrid materials and polymer electrolytes for electrochromic device applications. Adv Mater 24(30):4071–4096
Rosseinsky DR, Mortimer RJ (2001) Electrochromic systems and the prospects for devices. Adv Mater 13(11):783–793
Arias AC, MacKenzie JD, McCulloch I, Rivnay J, Salleo A (2010) Materials and applications for large area electronics: solution-based approaches. Chem Rev 110(1):3–24
Argun AA, Aubert P-H, Thompson BC, Schwendeman I, Gaupp CL, Hwang J, Pinto NJ, Tanner DB, MacDiarmid AG, Reynolds JR (2004) Multicolored electrochromism in polymers: structures and devices. Chem Mater 16(23):4401–4412
Krebs FC (2008) Electrochromic displays: the new black. Nat Mater 7(10):766–767
Yang Y, Li G (2015) Progress in high-efficient solution process organic photovoltaic devices. Springer, Berlin
Christoph B, Ullrich S, Vladimir D (2014) Organic photovoltaics: materials, device physics, and manufacturing technologies, 2nd Edn. Wiley-VCH, Hoboken
Liu X, Li Q, Li Y, Gong X, Su SJ, Cao Y (2014) Indacenodithiophene core-based small molecules with tunable side chains for solution-processed bulk heterojunction solar cells. J Mater Chem A 2(11):4004–4013
Dang D, Chen W, Himmelberger S, Tao Q, Lundin A, Yang R, Zhu W, Salleo A, Müller C, Wang E (2014) Enhanced photovoltaic performance of indacenodithiophene-quinoxaline copolymers by side-chain modulation. Adv Energy Mater 4(15):1400680
Chen K-S, Zhang Y, Yip H-L, Sun Y, Davies JA, Ting C, Chen C-P, Jen AKY (2011) Highly efficient indacenodithiophene-based polymeric solar cells in conventional and inverted device configurations. Org Electron 12(5):794–801
Sun Y, Chien S-C, Yip H-L, Chen K-S, Zhang Y, Davies JA, Chen F-C, Lin B, Jen AKY (2012) Improved thin film morphology and bulk-heterojunction solar cell performance through systematic tuning of the surface energy of conjugated polymers. J Mater Chem 22(12):5587–5595
Yong W, Zhang M, Xin X, Li Z, Wu Y, Guo X, Yang Z, Hou J (2013) Solution-processed indacenodithiophene-based small molecule for bulk heterojunction solar cells. J Mater Chem A 1(45):14214–14220
Zhang Y, Zou J, Yip H-L, Chen K-S, Zeigler DF, Sun Y, Jen AKY (2011) Indacenodithiophene and quinoxaline-based conjugated polymers for highly efficient polymer solar cells. Chem Mater 23(9):2289–2291
Bronstein H, Frost JM, Hadipour A, Kim Y, Nielsen CB, Ashraf RS, Rand BP, Watkins S, McCulloch I (2013) Effect of fluorination on the properties of a donor-acceptor copolymer for use in photovoltaic cells and transistors. Chem Mater 25(3):277–285
Bronstein H, Leem DS, Hamilton R, Woebkenberg P, King S, Zhang W, Ashraf RS, Heeney M, Anthopoulos TD, de Mello J, McCulloch I (2011) Indacenodithiophene-co-benzothiadiazole copolymers for high performance solar cells or transistors via alkyl chain optimization. Macromolecules 44(17):6649–6652
Sun Y, Chien S-C, Yip H-L, Zhang Y, Chen K-S, Zeigler DF, Chen F-C, Lin B, Jen AKY (2011) Chemically doped and cross-linked hole-transporting materials as an efficient anode buffer layer for polymer solar cells. Chem Mater 23(22):5006–5015
Chen C-P, Chan S-H, Chao T-C, Ting C, Ko B-T (2008) Low-bandgap poly(thiophene-phenylene-thiophene) derivatives with broaden absorption spectra for use in high-performance bulk-heterojunction polymer solar cells. J Am Chem Soc 130(38):12828–12833
Wang M, Hu X, Liu L, Duan C, Liu P, Ying L, Huang F, Cao Y (2013) Design and synthesis of copolymers of indacenodithiophene and naphtho 1,2-c:5,6-c bis(1,2,5-thiadiazole) for polymer solar cells. Macromolecules 46(10):3950–3958
Zhang Y, Zou J, Yip H-L, Chen K-S, Davies JA, Sun Y, Jen AKY (2011) Synthesis, characterization, charge transport, and photovoltaic properties of dithienobenzoquinoxaline- and dithienobenzopyridopyrazine-based conjugated polymers. Macromolecules 44(12):4752–4758
Chan S-H, Chen C-P, Chao T-C, Ting C, Lin C-S, Ko B-T (2008) Synthesis, characterization, and photovoltaic properties of novel semiconducting polymers with thiophene-phenylene-thiophene (TPT) as coplanar units. Macromolecules 41(15):5519–5526
Zhang W, Smith J, Watkins SE, Gysel R, McGehee M, Salleo A, Kirkpatrick J, Ashraf S, Anthopoulos T, Heeney M, McCulloch I (2010) Indacenodithiophene semiconducting polymers for high-performance, air-stable transistors. J Am Chem Soc 132(33):11437–11439
Neo WT, Ye Q, Lin TT, Chua SJ, Xu J (2015) 4,9-Dihydro-s-indaceno[1,2-b:5,6-b’]dithiophene-embedded electrochromic conjugated polymers with high coloration efficiency and fast coloration time. Sol Energy Mater Sol Cells 136:92–99
Gunbas G, Toppare L (2012) Electrochromic conjugated polyheterocycles and derivatives-highlights from the last decade towards realization of long lived aspirations. Chem Commun 48(8):1083–1101
Gaupp CL, Zong K, Schottland P, Thompson BC, Thomas CA, Reynolds JR (2000) Poly(3,4-ethylenedioxypyrrole): organic electrochemistry of a highly stable electrochromic polymer. Macromolecules 33(4):1132–1133
Gaupp CL, Welsh DM, Reynolds JR (2002) Poly(ProDOT-Et2): a high-contrast, high-coloration efficiency electrochromic polymer. Macromol Rapid Commun 23(15):885–889
Giglioti M, Trivinho-Strixino F, Matsushima JT, Bulhões LOS, Pereira EC (2004) Electrochemical and electrochromic response of poly(thiophene-3-acetic acid) films. Sol Energy Mater Sol Cells 82(3):413–420
Shin H, Kim Y, Bhuvana T, Lee J, Yang X, Park C, Kim E (2012) Color combination of conductive polymers for black electrochromism. ACS Appl Mater Interf 4(1):185–191
Ah CS, Song J, Cho SM, Kim T-Y, Kim HN, Oh JY, Chu HY, Ryu H (2015) Double-layered black electrochromic device with a single electrode and long-term bistability. Bull Korean Chem Soc 36(2):548–552
Neo WT, Cho CM, Song J, Chin JM, Wang X, He C, Chan HSO, Xu J (2013) Solution-processable multicolored dithienothiophene-based conjugated polymers for electrochromic applications. Eur Poly J 49(9):2446–2456
Aubert P-H, Knipper M, Groenendaal L, Lutsen L, Manca J, Vanderzande D (2004) Copolymers of 3,4-Ethylenedioxythiophene and of pyridine alternated with fluorene or phenylene units: synthesis, optical properties, and devices. Macromolecules 37(11):4087–4098
Sun Y, Chien S-C, Yip H-L, Zhang Y, Chen K-S, Zeigler DF, Chen F-C, Lin B, Jen AKY (2011) High-mobility low-bandgap conjugated copolymers based on indacenodithiophene and thiadiazolo[3,4-c]pyridine units for thin film transistor and photovoltaic applications. J Mater Chem 21(35):13247–13255
Beaujuge PM, Ellinger S, Reynolds JR (2008) Spray processable green to highly transmissive electrochromics via chemically polymerizable donor-acceptor heterocyclic pentamers. Adv Mater 20(14):2772–2776
Ye Q, Chang J, Huang KW, Shi X, Wu J, Chi C (2013) Cyanated diazatetracene diimides with ultrahigh electron affinity for n-channel field effect transistors. Organ Lett 15(6):1194–1197
Ye Q, Neo WT, Lin T, Song J, Yan H, Zhou H, Shah KW, Chua SJ, Xu J (2015) Pyrrolophthalazine dione (PPD)-based donor-acceptor polymers as high performance electrochromic materials. Polym Chem 6:1487–1494
Acknowledgements
The authors would like to thank the Agency for Science, Technology and Research (A*STAR) and Minister of National Development (MND) for financial support (Grant No.: 1321760011). The TD-DFT calculations were supported by the A*STAR computational resource centre through the use of its high-performance computing facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cho, C.M., Ye, Q., Neo, W.T. et al. Red-to-black electrochromism of 4,9-dihydro-s-indaceno[1,2-b:5,6-b’]dithiophene-embedded conjugated polymers. J Mater Sci 50, 5856–5864 (2015). https://doi.org/10.1007/s10853-015-9135-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9135-5