Abstract
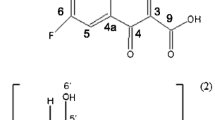
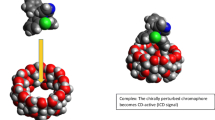
The enantiomer-specific characterization of ofloxacin–cyclodextrin complexes was carried out by a set of complementary analytical techniques. The apparent stability constants of the ofloxacin enantiomers with 20 different cyclodextrins at two different pH values were determined to achieve good resolution capillary electrophoresis enantioseparation either to establish enantioselective drug analysis assay, or to interpret and design improved host–guest interactions at the molecular level. The cyclodextrins studied differed in the nature of substituents, degree of substitution (DS), charge and purity, allowing a systematic test of these properties on the complexation. The seven-membered beta-cyclodextrin and its derivatives were found to be the most suitable hosts. Highest stability and best enantioseparation were observed for the carboxymethylated-beta-cyclodextrin (DS ~ 3.5). The effect of substitution pattern (SP) was investigated by molecular modeling, verifying that SP greatly affects the complex stability. Induced circular dichroism was observed and found especially significant on carboxymethylated-beta-cyclodextrin. The complex stoichiometry and the geometry of the inclusion complexes were determined by 1H NMR spectroscopy, including 2D ROESY techniques. Irrespective of the kind of cyclodextrin, the complexation ratio was found to be 1:1. The alfa-cyclodextrin cavity can accommodate the oxazine ring only, whereas the whole tricyclic moiety can enter the beta- and gamma-cyclodextrin cavities. These equilibrium and structural information offer molecular basis for improved drug formulation.








Similar content being viewed by others
References
Bolon, M.K.: The newer fluoroquinolones. Med. Clin. N. Am. 95, 793–817 (2011)
Andersson, M.I., MacGowan, A.P.: Development of the quinolones. J. Antimicrob. Chemother. 51, 1–11 (2003)
Ev Lda, S., Schapoval, E.E.: Microbiological assay for determination of ofloxacin injection. J. Pharm. Biomed. Anal. 27, 91–96 (2002)
Fujimoto, T., Mitsuhashi, S.: In vitro antibacterial activity of DR-3355, the S-(−)-isomer of ofloxacin. Chemotherapy 36, 268–276 (1990)
Morrissey, I., Hoshino, K., Sato, K., Yoshida, A., Hayakawa, I., Bures, M.G., Shen, L.L.: Mechanism of differential activities of ofloxacin enantiomers. Antimicrob. Agents Chemother. 40, 1775–1784 (1996)
US Pharmacopaea vol. USP-35-NF-30 (2012)
Bi, W., Tian, M., Row, K.H.: Chiral separation and determination of ofloxacin enantiomers by ionic liquid-assisted ligand-exchange chromatography. Analyst 136, 379–387 (2011)
Zeng, S., Zhong, J., Pan, L., Li, Y.: High-performance liquid chromatography separation and quantitation of ofloxacin enantiomers in rat microsomes. J. Chromatogr. B 728, 151–155 (1999)
Wong, F.A., Juzwin, S.J., Flor, S.C.: Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J. Pharm. Biomed. Anal. 15, 765–771 (1997)
de Boer, T., Mol, R., de Zeeuw, R.A., de Jong, G.J., Ensing, K.: Enantioseparation of ofloxacin in urine by capillary electrokinetic chromatography using charged cyclodextrins as chiral selectors and assessment of enantioconversion. Electrophoresis 22, 1413–1418 (2001)
Zhou, S., Ouyang, J., Baeyens, W.R., Zhao, H., Yang, Y.: Chiral separation of four fluoroquinolone compounds using capillary electrophoresis with hydroxypropyl-beta-cyclodextrin as chiral selector. J. Chromatogr. A 1130, 296–301 (2006)
Awadallah, B., Schmidt, P.C., Wahl, M.A.: Quantitation of the enantiomers of ofloxacin by capillary electrophoresis in the parts per billion concentration range for in vitro drug absorption studies. J. Chromatogr. A 988, 135–143 (2003)
Horstkotter, C., Blaschke, G.: Stereoselective determination of ofloxacin and its metabolites in human urine by capillary electrophoresis using laser-induced fluorescence detection. J. Chromatogr. B 754, 169–178 (2001)
Szejtli, J.: Cyclodextrin technology. Kluwer Academic, Dordrecht (1988)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Sohajda, T., Varga, E., Ivanyi, R., Fejos, I., Szente, L., Noszal, B., Beni, S.: Separation of vinca alkaloid enantiomers by capillary electrophoresis applying cyclodextrin derivatives and characterization of cyclodextrin complexes by nuclear magnetic resonance spectroscopy. J. Pharm. Biomed. Anal. 53, 1258–1266 (2010)
Challa, R., Ahuja, A., Ali, J., Khar, R.K.: Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech 6, E329–E357 (2005)
Hafner, V., Czock, D., Burhenne, J., Riedel, K.D., Bommer, J., Mikus, G., Machleidt, C., Weinreich, T., Haefeli, W.E.: Pharmacokinetics of sulfobutylether-beta-cyclodextrin and voriconazole in patients with end-stage renal failure during treatment with two hemodialysis systems and hemodiafiltration. Antimicrob. Agents Chemother. 54, 2596–2602 (2010)
Loftsson, T., Duchene, D.: Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 329, 1–11 (2007)
Buschmann, H.J., Schollmeyer, E.: New textile applications of cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 40, 169–172 (2001)
Koester, L.S., Guterres, S.S., Le Roch, M., Eifler-Lima, V.L., Zuanazzi, J.A., Bassani, V.L.: Ofloxacin/beta-cyclodextrin complexation. Drug Dev. Ind. Pharm. 27, 533–540 (2001)
Jinxia Li, X.Z.: Preparation and characterization of the inclusion complex of ofloxacin with beta-CD and HP-beta-CD. J. Incl. Phenom. Macrocycl. Chem. 69, 173–179 (2011)
Rusu, A., Toth, G., Szocs, L., Kokosi, J., Kraszni, M., Gyeresi, A., Noszal, B.: Triprotic site-specific acid-base equilibria and related properties of fluoroquinolone antibacterials. J. Pharm. Biomed. Anal. 66, 50–57 (2012)
Pop, M.M., Goubitz, K., Borodi, G., Bogdan, M., De Ridder, D.J., Peschar, R., Schenk, H.: Crystal structure of the inclusion complex of beta-cyclodextrin with mefenamic acid from high-resolution synchrotron powder-diffraction data in combination with molecular-mechanics calculations. Acta Crystallogr. B 58, 1036–1043 (2002)
Schmidt, A.K., Cottaz, S., Driguez, H., Schulz, G.E.: Structure of cyclodextrin glycosyltransferase complexed with a derivative of its main product beta-cyclodextrin. Biochemistry 37, 5909–5915 (1998)
Matsumoto, N., Yamada, M., Kurakata, Y., Yoshida, H., Kamitori, S., Nishikawa, A., Tonozuka, T.: Crystal structures of open and closed forms of cyclo/maltodextrin-binding protein. FEBS J. 276, 3008–3019 (2009)
Gyimesi, J., Szökő, É., Magyar, K., Barcza, L.: Determination of drug-cyclodextrin binding constants by capillary zone electrophoresis. J. Incl. Phenom. Macrocycl. Chem. 25, 253–256 (1996)
Rundlett, K.L., Armstrong, D.W.: Methods for the estimation of binding constants by capillary electrophoresis. Electrophoresis 18, 2194–2202 (1997)
Rundlett, K.L., Armstrong, D.W.: Examination of the origin, variation, and proper use of expressions for the estimation of association constants by capillary electrophoresis. J. Chromatogr. A 721, 173–186 (1996)
Shakalisava, Y., Regan, F.: Determination of association constants of inclusion complexes of steroid hormones and cyclodextrins from their electrophoretic mobility. Electrophoresis 27, 3048–3056 (2006)
Chen, Z., Weber, S.G.: Determination of binding constants by affinity capillary electrophoresis, electrospray ionization mass spectrometry and phase-distribution methods. Trends Anal. Chem. 27, 738–748 (2008)
Wren, S.A., Rowe, R.C.: Theoretical aspects of chiral separation in capillary electrophoresis III. Application to beta-blockers. J. Chromatogr. 635, 113–118 (1993)
Liu, X., Lin, H.S., Thenmozhiyal, J.C., Chan, S.Y., Ho, P.C.: Inclusion of acitretin into cyclodextrins: phase solubility, photostability, and physicochemical characterization. J. Pharm. Sci. 92, 2449–2457 (2003)
Mura, P., Bettinetti, G., Melani, F., Manderioli, A.: Interaction between naproxen and chemically modified β-cyclodextrins in the liquid and solid state. Eur. J. Pharm. Sci. 3, 347–355 (1995)
Yap, K.L., Liu, X., Thenmozhiyal, J.C., Ho, P.C.: Characterization of the 13-cis-retinoic acid/cyclodextrin inclusion complexes by phase solubility, photostability, physicochemical and computational analysis. Eur. J. Pharm. Sci. 25, 49–56 (2005)
Schonbeck, C., Westh, P., Madsen, J.C., Larsen, K.L., Stade, L.W., Holm, R.: Hydroxypropyl-substituted beta-cyclodextrins: influence of degree of substitution on the thermodynamics of complexation with tauroconjugated and glycoconjugated bile salts. Langmuir 26, 17949–17957 (2010)
Schonbeck, C., Westh, P., Madsen, J.C., Larsen, K.L., Stade, L.W., Holm, R.: Methylated beta-cyclodextrins: influence of degree and pattern of substitution on the thermodynamics of complexation with tauro- and glyco-conjugated bile salts. Langmuir 27, 5832–5841 (2011)
Allenmark, S.: Induced circular dichroism by chiral molecular interaction. Chirality 15, 409–422 (2003)
Bakirci, H., Zhang, X., Nau, W.M.: Induced circular dichroism and structural assignment of the cyclodextrin inclusion complexes of bicyclic azoalkanes. J. Org. Chem. 70, 39–46 (2005)
Matsuura, N., Takenaka, S., Tokura, N.: Formation of inclusion complexes of benzophenone derivatives: β-cyclodextrin studied by induced circular dichroism. J. Chem. Soc. 2, 1419–1421 (1977)
Harata, K., Uedaira, H.: Circular dichroism spectra of the β-cyclodextrin complex with naphthalene derivatives. Bull. Chem. Soc. Jpn. 48, 375–378 (1975)
Sohajda, T., Beni, S., Varga, E., Ivanyi, R., Racz, A., Szente, L., Noszal, B.: Characterization of aspartame-cyclodextrin complexation. J. Pharm. Biomed. Anal. 50, 737–745 (2009)
Acknowledgments
This work was supported by the National Scientific Research Fund of Hungary, OTKA K73804 and TÁMOP 4.2.1.B-09/1/KMR. This paper was also supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences (Sz. B.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tóth, G., Mohácsi, R., Rácz, Á. et al. Equilibrium and structural characterization of ofloxacin–cyclodextrin complexation. J Incl Phenom Macrocycl Chem 77, 291–300 (2013). https://doi.org/10.1007/s10847-012-0245-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-012-0245-2