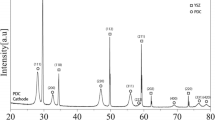
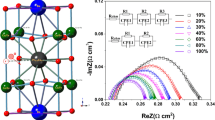
Abstract
Praseodymium-Cerium Oxide (PrxCe1-xO2−δ; PCO), a potential three way catalyst oxygen storage material and solid oxide fuel cell (SOFC) cathode, exhibits surprisingly high levels of oxygen nonstoichiometry, even under oxidizing (e.g. air) conditions, resulting in mixed ionic electronic conductivity (MIEC). In this study we examine the redox kinetics of dense PCO thin films using impedance spectroscopy, for x = 0.01, 0.10 and 0.20, over the temperature range of 550 to 670°C, and the oxygen partial pressure range of 10−4 to 1 atm O2. The electrode impedance was observed to be independent of electrode thickness and inversely proportional to electrode area, pointing to surface exchange rather than bulk diffusion limited kinetics. The large electrode capacitance (10−2F) was found to be consistent with an expected large electrochemically induced change in stoichiometry for x = 0.1 and x = 0.2 PCO. The PCO films showed surprisingly rapid oxygen exchange kinetics, comparable to other high performance SOFC cathode materials, from which values for the surface exchange coefficient, k q, were calculated. This study confirms the suitability of PCO as a model MIEC cathode material compatible with both zirconia and ceria based solid oxide electrolytes.











Similar content being viewed by others
References
M. Boaro, A. Trovarelli, J.H. Hwang, T.O. Mason, Solid State Ionics 147, 85 (2002)
S. Bernal, G. Blanco, J.J. Calvino, J.M. Gatica, J.A.P. Omil, J.M. Pintado, Top. Catal. 28, 31 (2004)
S. Haile, Mater. Today 6, 24 (2003)
B.C.H. Steele, Solid State Ionics 129, 95 (2000)
K. Eguchi, T. Setoguchi, T. Inoue, H. Arai, Solid State Ionics 52, 165 (1992)
S. Gupta, S.V.N.T. Kuchibhatla, M.H. Engelhard, V. Shutthanandan, P. Nachimuthu, W. Jiang, L.V. Saraf, S. Thevuthasan, S. Prasad, Sensor Actuator B Chem 139, 380 (2009)
K.L. Duncan, Y. Wang, S.R. Bishop, F. Ebrahimi, E.D. Wachsman, J. Am. Ceram. Soc. 89, 3162 (2006)
M. Boaro, C.D. Leitenburg, G. Dolcetti, A. Trovarelli, J. Catal. 193, 338 (2000)
S.R. Bishop, T.S. Stefanik, H.L. Tuller, Phys. Chem. Chem. Phys: PCCP 13, 10165 (2011)
T.S. Stefanik, Electrical Properties and Defect Structure of Praseodymium-Cerium Oxide Solid Solutions, in Department of Materials Science and Engineering (Massachusetts Institute of Technology, Cambridge, 2004)
S.R. Bishop, D. Chen, Y. Kuru, J.J. Kim, T.S. Stefanik, H.L. Tuller, ECS Trans. 33, 51 (2011)
S.R. Bishop, J.J. Kim, N. Thompson, D. Chen, Y. Kuru, T.S. Stefanik, H.L. Tuller, ECS Trans. 35, 1137 (2011)
R. Chiba, T. Komatsu, H. Orui, H. Taguchi, K. Nozawa, H. Arai, ECS Trans. 26, 333 (2010)
M.Y. Sinev, G.W. Graham, L.P. Haack, M. Shelef, J. Mater. Res. 11, 1960 (1996)
W. Jung, H.L. Tuller, Solid State Ionics 180, 843 (2009)
J. Fleig, F.S. Baumann, V. Brichzin, H.R. Kim, J. Jamnik, G. Cristiani, H.U. Habermeier, J. Maier, Fuel Cells 6, 284 (2006)
G.J. la O’, B. Yildiz, S. McEuen, Y. Shao-Horn, J. Electrochem. Soc. 154, B427 (2007)
K. Masato, Y. Masahiro, Bull. Chem. Soc. Jpn 72, 1427 (1999)
H.L. Tuller, S.R. Bishop, Annu. Rev. Mater. Res. 41, 369 (2011)
S.B. Adler, Chem. Rev. 104, 4791 (2004)
J. Fleig, Solid State Ionics 150, 181 (2002)
E.S. Thiele, L.S. Wang, T.O. Mason, S.A. Barnett, J. Vac. Sci. Tech. A: Vacuum, Surfaces, and Films 9, 1991 (1991)
P.S. Manning, J.D. Sirman, R.A. De Souza, J.A. Kilner, Solid State Ionics 100, 1 (1997)
T. Petrovsky, H.U. Anderson, V. Petrovsky, Mater. Res. Soc. Symp. Proc. 756, EE4.7.1 (2003)
W. Jung, H.L. Tuller, J. Electrochem. Soc. 155, B1194 (2008)
F.S. Baumann, J. Fleig, G. Cristiani, B. Stuhlhofer, H.-U. Habermeier, J. Maier, J. Electrochem. Soc. 154, B931 (2007)
F.S. Baumann, J. Fleig, H.-U. Habermeier, J. Maier, Solid State Ionics 177, 1071 (2006)
W.C. Chueh, S.M. Haile, Phys. Chem. Chem. Phys: PCCP 11, 8144 (2009)
N. Imanishi, T. Matsumura, Y. Sumiya, K. Yoshimura, A. Hirano, Y. Takeda, D. Mori, R. Kanno, Solid State Ionics 174, 245 (2004)
J. Maier, Physical Chemistry of Ionic Materials (Wiley, Chichester, 2004)
B.C.H. Steele, Solid State Ionics 75, 157 (1995)
R.A. De Souza, J.A. Kilner, Solid State Ionics 126, 153 (1999)
W. Jung, A New Model Describing Cathode Kinetics in Solid Oxide Fuel Cell: Model Thin Film SrTi 1-x Fe x O 3-δ Mixed Conducting Oxides – a case study, in Department of Materials Science and Engineering (Massachusetts Institute of Technology, Cambridge, 2010)
Acknowledgments
This work was supported by the National Science Foundation Materials World Network in collaboration with Prof. Moos, Universität Bayreuth, Germany under grant No. DMR-0908627. The authors thank Dr. WooChul Jung, MIT (now at Caltech), for providing constructive discussions, Mr. Jae Jin Kim for preparation of the PLD targets and the Center of Materials Science and Engineering (NSF-MRSEC) at MIT for use of its facilities. SRB recognizes partial support from I2CNER.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, D., Bishop, S.R. & Tuller, H.L. Praseodymium-cerium oxide thin film cathodes: Study of oxygen reduction reaction kinetics. J Electroceram 28, 62–69 (2012). https://doi.org/10.1007/s10832-011-9678-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10832-011-9678-z