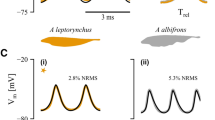
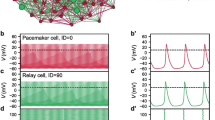
Abstract
Central pattern generators are characterized by a heterogeneous cellular composition, with different cell types playing distinct roles in the production and transmission of rhythmic signals. However, little is known about the functional implications of individual variation in the relative distributions of cells and their connectivity patterns. Here, we addressed this question through a combination of morphological data analysis and computational modeling, using the pacemaker nucleus of the weakly electric fish Apteronotus leptorhynchus as case study. A neural network comprised of 60–110 interconnected pacemaker cells and 15–30 relay cells conveying its output to electromotoneurons in the spinal cord, this nucleus continuously generates neural signals at frequencies of up to 1 kHz with high temporal precision. We systematically explored the impact of network size and density on oscillation frequencies and their variation within and across cells. To accurately determine effect sizes, we minimized the likelihood of complex dynamics using a simplified setup precluding differential delays. To identify natural constraints, parameter ranges were extended beyond experimentally recorded numbers of cells and connections. Simulations revealed that pacemaker cells have higher frequencies and lower within-population variability than relay cells. Within-cell precision and between-cells frequency synchronization increased with the number of pacemaker cells and of connections of either type, and decreased with relay cell count in both populations. Network-level frequency-synchronized oscillations occurred in roughly half of simulations, with maximized likelihood and firing precision within biologically observed parameter ranges. These findings suggest the structure of the biological pacemaker nucleus is optimized for generating synchronized sustained oscillations.







Similar content being viewed by others
References
Bennett, M. V. L. (1971). Electric organs. In W. S. Hoar & D. J. Randall (Eds.), Fish physiology, Vol. 5: Sensory systems and electric organs (pp. 347–491). Academic Press.
Borges, F. S., Protachevicz, P. R., Lameu, E. L., Bonetti, R. C., Iarosz, K. C., Caldas, I. L., Baptista, M. S., & Batista, A. M. (2017). Synchronised firing patterns in a random network of adaptive exponential integrate-and-fire neuron model. Neural Networks, 90, 1–7.
Brunel, N. (2000). Dynamics of sparsely connected networks of excitatory and inhibitory spiking neurons. Journal of Computational Neuroscience, 8, 183–208.
Bucher, D. (2009). Central pattern generators. In L. R. Squire (Ed.), Encyclopedia of neuroscience (pp. 691–700). Academic Press.
Bullock, T. H. (1969). Species differences in effect of electroreceptor input on electric organ pacemakers and other aspects of behavior in electric fish. Brain, Behavior and Evolution, 2, 85–118.
Bullock, T. H. (1970). The reliability of neurons. Journal of General Physiology, 55, 565–584.
Clay, J. R., & DeHaan, R. L. (1979). Fluctuations in interbeat interval in rhythmic heart-cell clusters: Role of membrane voltage noise. Biophysical Journal, 28, 377–389.
de Oliveira-Castro, G. (1955). Differentiated nervous fibers that constitute the electric organ of Sternarchus albifrons Linn. Anais Da Academia Brasileira De Ciências, 27, 557–564.
Dunlap, K. D., Thomas, P., & Zakon, H. H. (1998). Diversity of sexual dimorphism in electrocommunication signals and its androgen regulation in a genus of electric fish, Apteronotus. Journal of Comparative Physiology A, 183, 77–86.
Dye, J. (1988). An in vitro physiological preparation of a vertebrate communicatory behavior: Chirping in the weakly electric fish, Apteronotus. Journal of Comparative Physiology A, 163, 445–458.
Dye, J., & Heiligenberg, W. (1987). Intracellular recording in the medullary pacemaker nucleus of the weakly electric fish, Apteronotus, during modulatory behaviors. Journal of Comparative Physiology A, 161, 187–200.
Dye, J. C., & Meyer, J. H. (1986). Central control of the electric organ discharge in weakly electric fish. In T. H. Bullock & W. Heiligenberg (Eds.), Electroreception (pp. 71–102). John Wiley.
Elekes, K., & Szabo, T. (1985). Synaptology of the medullary command (pacemaker) nucleus of the weakly electric fish (Apteronotus leptorhynchus) with particular reference to comparative aspects. Experimental Brain Research, 60, 509–520.
Engler, G., & Zupanc, G. K. H. (2001). Differential production of chirping behavior evoked by electrical stimulation of the weakly electric fish, Apteronotus leptorhynchus. Journal of Comparative Physiology A, 187, 747–756.
Frigon, A. (2012). Central pattern generators of the mammalian spinal cord. The Neuroscientist, 18, 56–69.
Garcia, A. J., 3rd., Zanella, S., Koch, H., Doi, A., & Ramirez, J.-M. (2011). Networks within networks: The neuronal control of breathing. Progress in Brain Research, 188, 31–50.
Golomb, D., & Hansel, D. (2000). The number of synaptic inputs and the synchrony of large, sparse neuronal networks. Neural Computation, 12, 1095–1139.
Grabow, C., Grosskinsky, S., & Timme, M. (2011). Speed of complex network synchronization. European Physical Journal B, 84, 613–626.
Grabow, C., Hill, S. M., Grosskinsky, S., & Timme, M. (2010). Do small worlds synchronize fastest? EPL, 90, 48002.
Grillner, S. (2006). Biological pattern generation: The cellular and computational logic of networks in motion. Neuron, 52, 751–766.
Guertin, P. A. (2009). The mammalian central pattern generator for locomotion. Brain Research Reviews, 62, 45–56.
Gutierrez, G. J., & Marder, E. (2013). Rectifying electrical synapses can affect the influence of synaptic modulation on output pattern robustness. Journal of Neuroscience, 33, 13238–13248.
Harris-Warrick, R., Marder, E., Selverston, A. I., & Moulins, M. (1992). Dynamics biological networks. MIT Press.
Hartman, D., Lehotzky, D., Ilieş, I., Levi, M., & Zupanc, G. K. H. (2021). Modeling of sustained spontaneous network oscillations of a sexually dimorphic brainstem nucleus: The role of potassium equilibrium potential. Journal of Computational Neuroscience, 49, 419–439.
Heiligenberg, W., Metzner, W., Wong, C. J. H., & Keller, C. H. (1996). Motor control of the jamming avoidance response of Apteronotus leptorhynchus: Evolutionary changes of a behavior and its neuronal substrates. Journal of Comparative Physiology A, 179, 653–674.
Hines, M. L., & Carnevale, N. T. (1997). The NEURON simulation environment. Neural Computation, 9, 1179–1209.
Hines, M. L., & Carnevale, N. T. (2000). Expanding NEURON’s repertoire of mechanisms with NMODL. Neural Computation, 12, 995–1007.
Hines, M. L., Davison, A. P., & Muller, E. (2009). NEURON and Python. Frontiers in Neuroinformatics, 3, 1.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117, 500–544.
Hudson, A. E., Archila, S., & Prinz, A. A. (2010). Identifiable cells in the crustacean stomatogastric ganglion. Physiology, 25, 311–318.
Katz, P. S. (2016). Evolution of central pattern generators and rhythmic behaviours. Philosophical Transactions of the Royal Society B, 371, 20150057.
Kori, H., Kawamura, Y., & Masuda, N. (2012). Structure of cell networks critically determines oscillation regularity. Journal of Theoretical Biology, 297, 61–72.
Lodi, M., Della Rossa, F., Sorrentino, F., & Storace, M. (2020). Analyzing synchronized clusters in neuron networks. Scientific Reports, 10, 16336.
Lucas, K. M., Warrington, J., Lewis, T. J., & Lewis, J. E. (2019). Neuronal dynamics underlying communication signals in a weakly electric fish: Implications for connectivity in a pacemaker network. Neuroscience, 401, 21–34.
Marder, E., & Bucher, D. (2001). Central pattern generators and the control of rhythmic movements. Current Biology, 11, R986–R996.
Moortgat, K. T., Bullock, T. H., & Sejnowski, T. J. (2000a). Precision of the pacemaker nucleus in a weakly electric fish: Network versus cellular influences. Journal of Neurophysiology, 83, 971–983.
Moortgat, K. T., Bullock, T. H., & Sejnowski, T. J. (2000b). Gap junction effects on precision and frequency of a model pacemaker network. Journal of Neurophysiology, 83, 984–997.
Moortgat, K. T., Keller, C. H., Bullock, T. H., & Sejnowski, T. J. (1998). Submicrosecond pacemaker precision is behaviorally modulated: the gymnotiform electromotor pathway. Proceedings of the National Academy of Sciences U.S.A., 95, 4684–4689.
Selverston, A. I. (2010). Invertebrate central pattern generator circuits. Philosophical Transactions of the Royal Society B, 365, 2329–2345.
Sîrbulescu, R. F., Ilieş, I., & Zupanc, G. K. H. (2014). Quantitative analysis reveals dominance of gliogenesis over neurogenesis in an adult brainstem oscillator. Developmental Neurobiology, 74, 934–952.
Stiefel, K. M., & Ermentrout, G. B. (2016). Neurons as oscillators. Journal of Neurophysiology, 116, 2950–2960.
Tattini, L., Olmi, S., & Torcini, A. (2012). Coherent periodic activity in excitatory Erdös-Renyi neural networks: The role of network connectivity. Chaos, 22, 023133.
Tönjes, R., Masuda, N., & Kori, H. (2010). Synchronization transition of identical phase oscillators in a directed small-world network. Chaos, 20, 033108.
Vasalou, C., Herzog, E. D., & Henson, M. A. (2009). Small-world network models of intercellular coupling predict enhanced synchronization in the suprachiasmatic nucleus. Journal of Biological Rhythms, 24, 243–254.
Waxman, S. G., Pappas, G. D., & Bennett, M. V. L. (1972). Morphological correlates of functional differentiation of nodes of Ranvier along single fibers in the neurogenic electric organ of the knife fish Sternarchus. Journal of Cell Biology, 53, 210–224.
White, J. A., Chow, C. C., Ritt, J., Soto-Treviño, C., & Kopell, N. (1998). Synchronization and oscillatory dynamics in heterogeneous, mutually inhibited neurons. Journal of Computational Neuro-science, 5, 5–16.
Yamamoto, T., Maler, L., Hertzberg, E. L., & Nagy, J. I. (1989). Gap junction protein in weakly electric fish (Gymnotide [sic]): Immuno-histochemical localization with emphasis on structures of the electrosensory system. Journal of Comparative Neurology, 289, 509–536.
Zupanc, G. K. H. (2018). Electrocommunication. In J. P. Stein (Ed.), Reference module in neuroscience and biobehavioral psychology (pp. 1–13). Elsevier.
Zupanc, G. K. H., Amaro, S. M., Lehotzky, D., Zupanc, F. B., & Leung, N. Y. (2019). Glia-mediated modulation of extracellular potassium concentration determines the sexually dimorphic output frequency of a model brainstem oscillator. Journal of Theoretical Biology, 471, 117–124.
Zupanc, G. K. H., & Bullock, T. H. (2005). From electrogenesis to electroreception: An overview. In T. H. Bullock, C. D. Hopkins, A. N. Popper, & R. R. Fay (Eds.), Electroreception (pp. 5–46). Springer Science + Business Media.
Zupanc, G. K. H., Ilieş, I., Sîrbulescu, R. F., & Zupanc, M. M. (2014). Large-scale identification of proteins involved in the development of a sexually dimorphic behavior. Journal of Neurophysiology, 111, 1646–1654.
Zupanc, G. K. H., & Maler, L. (1993). Evoked chirping in the weakly electric fish Apteronotus leptorhynchus: A quantitative biophysical analysis. Canadian Journal of Zoology, 71, 2301–2310.
Zupanc, G. K. H., Sîrbulescu, R. F., Nichols, A., & Ilies, I. (2006). Electric interactions through chirping behavior in the weakly electric fish, Apteronotus leptorhynchus. Journal of Comparative Physiology A, 192, 159–173.
Acknowledgements
The code file implementing the NEURON model is available in a public GitHub repository at the following link: https://github.com/LaboratoryOfNeurobiology/modeling-heterogeneous-cpg. We would like to thank Dr. Ruxandra F. Sîrbulescu for providing the confocal image of the A. leptorhynchus Pn shown in Figure 1A, and for helpful discussions. This research was supported by Grant 1946910 from the National Science Foundation (GKHZ).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Action Editor: Catherine E Carr
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ilieş, I., Zupanc, G.K.H. Computational modeling predicts regulation of central pattern generator oscillations by size and density of the underlying heterogenous network. J Comput Neurosci 51, 87–105 (2023). https://doi.org/10.1007/s10827-022-00835-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-022-00835-7