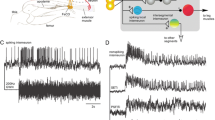
Abstract
Directed information transfer measures are increasingly being employed in modeling neural system behavior due to their model-free approach, applicability to nonlinear and stochastic signals, and the potential to integrate repetitions of an experiment. Intracellular physiological recordings of graded synaptic potentials provide a number of additional challenges compared to spike signals due to non-stationary behaviour generated through extrinsic processes. We therefore propose a method to overcome this difficulty by using a preprocessing step based on Singular Spectrum Analysis (SSA) to remove nonlinear trends and discontinuities. We apply the method to intracellular recordings of synaptic responses of identified motor neurons evoked by stimulation of a proprioceptor that monitors limb position in leg of the desert locust. We then apply normalized delayed transfer entropy measures to neural responses evoked by displacements of the proprioceptor, the femoral chordotonal organ, that contains sensory neurones that monitor movements about the femoral-tibial joint. We then determine the consistency of responses within an individual recording of an identified motor neuron in a single animal, between repetitions of the same experiment in an identified motor neurons in the same animal and in repetitions of the same experiment from the same identified motor neuron in different animals. We found that delayed transfer entropy measures were consistent for a given identified neuron within and between animals and that they predict neural connectivity for the fast extensor tibiae motor neuron.








Similar content being viewed by others
Notes
The code used in the analysis is publicly available at https://github.com/lablps/JCNS2017. Further information may be obtained by contacting the authors.
References
Angarita-Jaimes, N., Dewhirst, O. P., Simpson, D. M., Kondoh, Y., Allen, R., & Newland, P. L. (2012). The dynamics of analogue signaling in local networks controlling limb movement. European Journal of Neuroscience, 36(9), 3269–3282.
Barnett, L., & Seth, A. K. (2011). Behaviour of Granger causality under filtering: theoretical invariance and practical application. Journal of Neuroscience Methods, 201(2), 404–419.
Barnett, L., Barrett, A. B., & Seth, A. K. (2009). Granger causality and transfer entropy are equivalent for Gaussian variables. Physical Review Letters, 103(23), 238701.
Bässler, U. (1993). The femur-tibia control system of stick insects—a model system for the study of the neural basis of joint control. Brain Research Reviews, 18(2), 207–226.
Benda, J., Longtin, A., & Maler, L. (2005). Spike-frequency adaptation separates transient communication signals from background oscillations. Journal of Neuroscience, 25(9), 2312–2321.
Burrows, M. (1987). Parallel processing of proprioceptive signals by spiking local interneurons and motor neurons in the locust. Journal of Neuroscience, 7(4), 1064–1080.
Burrows, M. (1988). Responses of spiking local interneurones in the locust to proprioceptive signals from the femoral chordotonal organ. Journal of Comparative Physiology A, 164(2), 207–217.
Burrows, M. (1996). The Neurobiology of an Insect Brain. Oxford: Oxford University Press.
Buschmann, T., Ewald, A., von Twickel, A., & Büschges, A. (2015). Controlling legs for locomotion—Insights from robotics and neurobiology. Bioinspiration & Biomimetics, 10(4), 041001.
Cook, D. L., Schwindt, P. C., Grande, L. A., & Spain, W. J. (2003). Synaptic depression in the localization of sound. Nature, 421(6918), 66–70.
Dewhirst, O. P., Angarita-Jaimes, N., Simpson, D. M., Allen, R., & Newland, P. L. (2013). A system identification analysis of neural adaptation dynamics and nonlinear responses in the local reflex control of locust hind limbs. Journal of Computational Neuroscience, 34(1), 39–58.
Dolan, K. T., & Spano, M. L. (2001). Surrogate for nonlinear time series analysis. Physical Review E, 64(4), 046128.
Ebeling, W. (2002). Entropies and predictability of nonlinear processes and time series. In International Conference on Computational Science (pp. 1209–1217). Berlin Heidelberg: Springer.
Endo, W., Santos, F. P., Simpson, D., Maciel, C. D., & Newland, P. L. (2015). Delayed mutual information infers patterns of synaptic connectivity in a proprioceptive neural network. Journal of Computational Neuroscience, 38(2), 427–438.
Faes, L., & Porta, A. (2014). Conditional entropy-based evaluation of information dynamics in physiological systems. In Directed information measures in neuroscience (pp. 61–86). Berlin Heidelberg: Springer.
Field, L. H., & Burrows, M. (1982). Reflex effects of the femoral chordotonal organ upon leg motor neurones of the locust. Journal of Experimental Biology, 101(1), 265–285.
Florin, E., Gross, J., Pfeifer, J., Fink, G. R., & Timmermann, L. (2010). The effect of filtering on Granger causality based multivariate causality measures. NeuroImage, 50(2), 577–588.
Gamble, E. R., & DiCaprio, R. A. (2003). Nonspiking and spiking proprioceptors in the crab: white noise analysis of spiking CB-chordotonal organ afferents. Journal of Neurophysiology, 89(4), 1815–1825.
Golyandina, N., & Zhigljavsky, A. (2013). Singular Spectrum Analysis for time series. Berlin Heidelberg: Springer-Verlag. http://www.springer.com/br/book/9783642349126.
Gourevitch, B., & Eggermont, J. J. (2007). Evaluating information transfer between auditory cortical neurons. Journal of Neurophysiology, 97(3), 2533–2543.
Grazzini, J. (2012). Analysis of the emergent properties: stationarity and ergodicity. Journal of Artificial Societies and Social Simulation, 15(2), 7.
Grzegorczyk, M., & Husmeier, D. (2009). Non-stationary continuous dynamic Bayesian networks. Advances in Neural Information Processing Systems, 682–690.
Hassani, H. (2007). Singular spectrum analysis: methodology and comparison. Journal of Data Science, 5(2), 239–257.
Hlaváčková-Schindler, K., Paluš, M., Vejmelka, M., & Bhattacharya, J. (2007). Causality detection based on information-theoretic approaches in time series analysis. Physics Reports, 441(1), 1–46.
Ince, R. A., Mazzoni, A., Bartels, A., Logothetis, N. K., & Panzeri, S. (2012). A novel test to determine the signi cancer of neural selectivity to single and multiple potentially correlated stimulus features. Journal of Neuroscience Methods, 210(1), 49–65.
Kaiser, A., & Schreiber, T. (2002). Information transfer in continuous processes. Physica D: Nonlinear Phenomena, 166(1), 43–62.
Kantz, H., & Schreiber, T. (2004). Nonlinear time series analysis. New York: Cambridge University Press. http://dl.acm.org/citation.cfm?id=289372.
Kittmann, R. (1997). Neural mechanisms of adaptive gain control in a joint control loop: muscle force and motoneuronal activity. Journal of Experimental Biology, 200(9), 1383–1402.
Knoblauch, A., & Sommer, F. T. (2016). Structural plasticity, effectual connectivity, and memory in cortex. Frontiers in Neuroanatomy, 10, 63.
Kondoh, Y., Okuma, J., & Newland, P. L. (1995). Dynamics of neurons controlling movements of a locust hind leg: Wiener kernel analysis of the responses of proprioceptive afferents. Journal of Neurophysiology, 73(5), 1829–1842.
Kovač, M. (2014). The bioinspiration design paradigm: A perspective for soft robotics. Soft Robotics, 1(1), 28–37.
Lee, J., Nemati, S., Silva, I., Edwards, B. A., Butler, J. P., & Malhotra, A. (2012). Transfer entropy estimation and directional coupling change detection in biomedical time series. Biomedical Engineering Online, 11(1), 19.
Meruelo, A. C., Simpson, D. M., Veres, S. M., & Newland, P. L. (2016). Improved system identification using artificial neural networks and analysis of individual differences in responses of an identified neuron. Neural Networks, 75, 56–65.
Nawrot, M. P. (2010). Analysis and interpretation of interval and count variability in neural spike trains. In Analysis of parallel spike trains (pp. 37–58). Boston: Springer. https://link.springer.com/chapter/10.1007%2F978-1-4419-5675-0_3.
Newland, P. L. (1991). Morphology and somatotopic organisation of the central projections of afferents from tactile hairs on the hind leg of the locust. Journal of Comparative Neurology, 312(4), 493–508.
Newland, P. L., & Kondoh, Y. (1997a). Dynamics of neurons controlling movements of a locust hind leg II. Flexor tibiae motor neurons. Journal of Neurophysiology, 77(4), 1731–1746.
Newland, P. L., & Kondoh, Y. (1997b). Dynamics of neurons controlling movements of a locust hind leg III. Extensor tibiae motor neurons. Journal of Neurophysiology, 77(6), 3297–3310.
Orlandi, J. G., Stetter, O., Soriano, J., Geisel, T., & Battaglia, D. (2014). Transfer entropy reconstruction and labeling of neuronal connections from simulated calcium imaging. PloS One, 9(6), e98842.
Palus, M., & Novotná, D. (1998). Detecting modes with nontrivial dynamics embedded in colored noise: Enhanced Monte Carlo SSA and the case of climate oscillations. Physics Letters A, 248(2), 191–202.
Pampu, N. C., Vicente, R., Muresan, R. C., Priesemann, V., Siebenhuhner, F., & Wibral, M. (2013, July). Transfer entropy as a tool for reconstructing interaction delays in neural signals. In Signals, Circuits and Systems (ISSCS), 2013 International Symposium on (pp. 1–4). IEEE.
Prescott, S. A., & Sejnowski, T. J. (2008). Spike-rate coding and spike-time coding are affected oppositely by different adaptation mechanisms. Journal of Neuroscience, 28(50), 13649–13661.
Schreiber, T. (2000). Measuring information transfer. Physical Review Letters, 85(2), 461.
Schreiber, T., & Schmitz, A. (2000). Surrogate time series. Physica D: Nonlinear Phenomena, 142(3), 346–382.
Schroeder, K. E., Irwin, Z. T., Gaidica, M., Bentley, J. N., Patil, P. G., Mashour, G. A., & Chestek, C. A. (2016). Disruption of corticocortical information transfer during ketamine anesthesia in the primate brain. NeuroImage, 134, 459–465.
Silchenko, A. N., Adamchic, I., Pawelczyk, N., Hauptmann, C., Maarouf, M., Sturm, V., & Tass, P. A. (2010). Data-driven approach to the estimation of connectivity and time delays in the coupling of interacting neuronal subsystems. Journal of Neuroscience Methods, 191(1), 32–44.
Smith, V. A., Yu, J., Smulders, T. V., Hartemink, A. J., & Jarvis, E. D. (2006). Computational inference of neural information flow networks. PLoS Computational Biology, 2(11), e161.
Therrien, C. W. (1992). Discrete random signals and statistical signal processing. Englewood Cliffs: Prentice Hall.
Vautard, R., Yiou, P., & Ghil, M. (1992). Singular-spectrum analysis: A toolkit for short, noisy chaotic signals. Physica D: Nonlinear Phenomena, 58(1), 95–126.
Venema, V., Ament, F., & Simmer, C. (2006). A stochastic iterative amplitude adjusted fourier transform algorithm with improved accuracy. Nonlinear Processes in Geophysics, 13(3), 321–328.
Vidal-Gadea, A. G., Jing, X., Simpson, D., Dewhirst, O. P., Kondoh, Y., Allen, R., & Newland, P. L. (2010). Coding characteristics of spiking local interneurons during imposed limb movements in the locust. Journal of Neurophysiology, 103(2), 603–615.
Vitanza, A., Patané, L., & Arena, P. (2015). Spiking neural controllers in multi-agent competitive systems for adaptive targeted motor learning. Journal of the Franklin Institute, 352(8), 3122–3143.
Watson, A. H., & Burrows, M. (1987). Immunocytochemical and pharmacological evidence for GABAergic spiking local interneurons in the locust. Journal of Neuroscience, 7(6), 1741–1751.
Wibral, M., Pampu, N., Priesemann, V., Siebenhühner, F., Seiwert, H., Lindner, M., & Vicente, R. (2013). Measuring information-transfer delays. PloS One, 8(2), e55809.
Wibral, M., Vicente, R., & Lindner, M. (2014). Transfer entropy in neuroscience. In Directed Information Measures in Neuroscience (pp. 3–36). Berlin Heidelberg: Springer.
Wilmer, A., de Lussanet, M., & Lappe, M. (2012). Time-delayed mutual information of the phase as a measure of functional connectivity. PloS One, 7(9), e44633.
Wollstadt, P., Martínez-Zarzuela, M., Vicente, R., Díaz-Pernas, F. J., & Wibral, M. (2014). Efficient transfer entropy analysis of non-stationary neural time series. PloS One, 9(7), e102833.
Yang, C., Jeannès, R. L. B., Faucon, G., & Shu, H. (2013). Detecting information flow direction in multivariate linear and nonlinear models. Signal Processing, 93(1), 304–312.
Acknowledgements
The authors would like to thank the Sao Paulo Research Foundations FAPESP (grant 2012/24272-7), CNPq (grant 475064/2013-5). PLN was supported by a PVE award from Science Without Borders (Brazil) and by a collaborative award from FAPEMIG-University of Southampton.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Simon R Schultz
Appendix A: Singular spectrum analysis
Appendix A: Singular spectrum analysis
The SSA algorithm is described as follows (Golyandina and Zhigljavsky 2013): consider a time-series Z T = (z 1, … , z T ). The embedding matrix of Z is obtained as:
where K = T − L + 1 and L are window lengths considered for calculation (L ≤ T/2). It is also important to note that Z is a Hankel matrix and has equal elements on the anti-diagonals. A singular value decomposition (SVD) value is be applied to the matrix ZZ ′, representing it as a sum of rank-one bi-orthogonal elementary matrices. We then denote λ 1, λ 2, … , λ L is as the eigenvalues of ZZ ′ in decreasing order of magnitude λ 1 ≥ … ≥ λ L ≥ 0 and P = (P 1, P 2, … , P L ) is the orthonormal system of the eigenvectors of ZZ ′ corresponding to these eigenvalues (Golyandina and Zhigljavsky 2013). We also define d as d = max(i, such that λ i > 0) = rank Z (in real data, we usually have d = min {L, K}).
The principal components (PCs) V i (i = 1 , … , d) of the embedding matrix are then obtained by \( {V}_i={\boldsymbol{Z}}^{\prime }{P}_i/\sqrt{\lambda_i} \), and thus the trajectory matrix can be written as Z = Z 1 + Z 2 + Z 3 + … + Z d , where \( {\boldsymbol{Z}}_i=\sqrt{\lambda_i}{P}_i{V_i}^{\prime}\left(i=1,\dots, d\right) \). These matrices have rank 1, and therefore are called elementary matrices (Hassani 2007).
The signal, then, can be reconstructed by selecting PCs according to their desired properties and then projecting them back to the original coordinates of the time-series. This is done by selecting and partitioning the indices i = 1 , … , d into disjoint subsets I 1 , I 2 , … , I m . Then, for a given subset I = {i 1, i 2, … , i Q } the corresponding resultant matrix Z I is defined as \( {\boldsymbol{Z}}_I={\boldsymbol{Z}}_{i_1}+{\boldsymbol{Z}}_{i_2}+\dots +{\boldsymbol{Z}}_{i_Q} \) . This also leads to the SVD decomposition being represented as \( \boldsymbol{Z}={\boldsymbol{Z}}_{I_1}+{\boldsymbol{Z}}_{I_2}+\dots +{\boldsymbol{Z}}_{I_m} \).
Diagonal averaging is then applied to a matrix \( {X}_{I_k} \) producing the reconstructed time-series \( {\overset{\sim }{\mathrm{Z}}}^{(k)}=\left({{\overset{\sim }{z}}_1}^{(k)},{{\overset{\sim }{z}}_2}^{(k)},\dots, {{\overset{\sim }{z}}_T}^{(k)}\right) \). In this way, the original series is decomposed into a sum of m reconstructed subseries:
With this, SSA can be used as a tool for time-series smoothing, extraction of trends and extraction of oscillatory components (Golyandina and Zhigljavsky 2013).
Rights and permissions
About this article
Cite this article
Santos, F.P., Maciel, C.D. & Newland, P.L. Pre-processing and transfer entropy measures in motor neurons controlling limb movements. J Comput Neurosci 43, 159–171 (2017). https://doi.org/10.1007/s10827-017-0656-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-017-0656-6