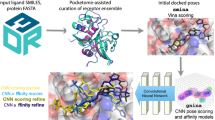
Abstract
We present the performances of our mathematical deep learning (MathDL) models for D3R Grand Challenge 4 (GC4). This challenge involves pose prediction, affinity ranking, and free energy estimation for beta secretase 1 (BACE) as well as affinity ranking and free energy estimation for Cathepsin S (CatS). We have developed advanced mathematics, namely differential geometry, algebraic graph, and/or algebraic topology, to accurately and efficiently encode high dimensional physical/chemical interactions into scalable low-dimensional rotational and translational invariant representations. These representations are integrated with deep learning models, such as generative adversarial networks (GAN) and convolutional neural networks (CNN) for pose prediction and energy evaluation, respectively. Overall, our MathDL models achieved the top place in pose prediction for BACE ligands in Stage 1a. Moreover, our submissions obtained the highest Spearman correlation coefficient on the affinity ranking of 460 CatS compounds, and the smallest centered root mean square error on the free energy set of 39 CatS molecules. It is worthy to mention that our method on docking pose predictions has significantly improved from our previous ones.









Similar content being viewed by others
Notes
This subcategory is the common list of ligand based and structure based scoring subcategories.
References
Gathiaka S, Liu S, Chiu M, Yang H, Stuckey JA, Kang YN, Delproposto J, Kubish G, Dunbar JB, Carlson HA et al (2016) D3r grand challenge 2015: evaluation of protein-ligand pose and affinity predictions. J Comput-Aided Mol Des 30(9):651–668
Gaieb Z, Liu S, Gathiaka S, Chiu M, Yang H, Shao C, Feher VA, Walters WP, Kuhn B, Rudolph MG et al (2018) D3r grand challenge 2: blind prediction of protein-ligand poses, affinity rankings, and relative binding free energies. J Comput-Aided Mol Des 32(1):1–20
Gaieb Z, Parks CD, Chiu M, Yang H, Shao C, Walters WP, Lambert MH, Nevins N, Bembenek SD, Ameriks MK et al (2019) D3r grand challenge 3: blind prediction of protein-ligand poses and affinity rankings. J Comput-Aided Mol Des 33(1):1–18
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31(2):455–461
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267(3):727–748
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, JK JKP, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. method and assessment of docking accuracy. J Med Chem 47:1739
Abagyan R, Totrov M, Kuznetsov D (1994) Icm-a new method for protein modeling and design: applications to docking and structure prediction from the distorted native conformation. J Comput Chem 15(5):488–506
Liu J, Wang R (2015) Classification of current scoring functions. J Chem Inf Model 55(3):475–482
Ortiz AR, Pisabarro MT, Gago F, Wade RC (1995) Prediction of drug binding affinities by comparative binding energy analysis. J Med Chem 38:2681–2691
Yin S, Biedermannova L, Vondrasek J, Dokholyan NV (2008) Medusascore: an acurate force field-based scoring function for virtual drug screening. J Chem Inf Model 48:1656–1662
Muegge I, Martin Y (1999) A general and fast scoring function for protein-ligand interactions: a simplified potential approach. J Med Chem 42(5):791–804
Velec HFG, Gohlke H, Klebe G (2005) Knowledge-based scoring function derived from small molecule crystal data with superior recognition rate of near-native ligand poses and better affinity prediction. J Med Chem 48:6296–6303
Zheng Z, Wang T, Li P, Merz KM Jr (2015) KECSA-Movable type implicit solvation model (KMTISM). J Chem Theor Comput 11:667–682
Huang SY, Zou X (2006) An iterative knowledge-based scoring function to predict protein-ligand interactions: I. derivation of interaction potentials. J Comput Chem 27:1865–1875
Verkhivker G, Appelt K, Freer ST, Villafranca JE (1995) Empirical free energy calculations of ligand-protein crystallographic complexes. i. Knowledge based ligand-protein interaction potentials applied to the prediction of human immunodeficiency virus protease binding affinity. Protein Eng 8:677–691
Eldridge MD, Murray CW, Auton TR, Paolini GV, Mee RP (1997) Empirical scoring functions: I. The development of a fast empirical scoring function to estimate the binding affinity of ligands in receptor complexes. J Comput-Aided Mol Des 11:425–445
Wang R, Lai L, Wang S (2002) Further development and validation of empirical scoring functions for structural based binding affinity prediction. J. Comput-Aided Mol. Des 16:11–26
Ballester PJ, Mitchell JBO (2010) A machine learning approach to predicting protein -ligand binding affinity with applications to molecular docking. Bioinformatics 26(9):1169–1175
Breiman L (2001) Random forests. Mach Learn 45(1):5–32
Li H, Leung K-S, Wong M-H, Ballester PJ (2014) Substituting random forest for multiple linear regression improves binding affinity prediction of scoring functions: cyscore as a case study. BMC Bioinform 15(1):1
Nguyen DD, Xiao T, Wang ML, Wei GW (2017) Rigidity strengthening: a mechanism for protein-ligand binding. J Chem Inf Model 57:1715–1721
Cang ZX, Wei GW (2018) Integration of element specific persistent homology and machine learning for protein-ligand binding affinity prediction. Int J Numer Method Biomed Eng. https://doi.org/10.1002/cnm.2914
Cang ZX, Wei GW (2017) TopologyNet: topology based deep convolutional and multi-task neural networks for biomolecular property predictions. PLOS Comput Biol 13(7):e1005690. https://doi.org/10.1371/journal.pcbi.1005690
Cang ZX, Mu L, Wei GW (2018) Representability of algebraic topology for biomolecules in machine learning based scoring and virtual screening. PLOS Comput Biol 14(1):e1005929. https://doi.org/10.1371/journal.pcbi.1005929
Nguyen DD, Wei G-W (2019) Dg-gl: differential geometry-based geometric learning of molecular datasets. Int J Numer Method Biomed Eng 35(3):e3179
Nguyen D, Wei G-W (2019) Agl-score: algebraic graph learning score for protein-ligand binding scoring, ranking, docking, and screening. J Chem Inf Model 59(7):3291–3304
Nguyen DD, Cang Z, Wu K, Wang M, Cao Y, Wei G-W (2019) Mathematical deep learning for pose and binding affinity prediction and ranking in d3r grand challenges. J Comput-Aided Mol Des 33(1):71–82
Wei GW (2010) Differential geometry based multiscale models. Bull Math Biol 72:1562–1622
Chen Z, Zhao S, Chun J, Thomas DG, Baker NA, Bates PB, Wei GW (2012) Variational approach for nonpolar solvation analysis. J Chem Phys 137:084101
Wang B, Wei G-W (2015) Parameter optimization in differential geometry based solvation models. J Chem Phys 143:134119
Chen D, Wei GW (2012) Quantum dynamics in continuum for proton transport III: generalized correlation. J Chem Phys 136:134109
Chen D, Wei GW (2012) Quantum dynamics in continuum for proton transport—generalized correlation. J Chem Phys 136:134109
Wei G-W, Zheng Q, Chen Z, Xia K (2012) Variational multiscale models for charge transport. SIAM Rev 54(4):699–754
Wei GW (2013) Multiscale, multiphysics and multidomain models I: basic theory. J Theor Comput Chem 12(8):1341006
Chen D, Wei GW (2013) Quantum dynamics in continuum for proton transport I: basic formulation. Commun Comput Phys 13:285–324
Feng X, Xia K, Tong Y, Wei G-W (2012) Geometric modeling of subcellular structures, organelles and large multiprotein complexes. Int J Numer Method Biomed Eng 28:1198–1223
Xia KL, Feng X, Tong YY, Wei GW (2014) Multiscale geometric modeling of macromolecules i: Cartesian representation. J Comput Phys 275:912–936
Mu L, Xia K, Wei G (2017) Geometric and electrostatic modeling using molecular rigidity functions. J Comput Appl Math 313:18–37
Nguyen DD, Wei GW (2017) The impact of surface area, volume, curvature and Lennard-Jones potential to solvation modeling. J Comput Chem 38:24–36
Kaczynski T, Mischaikow K, Mrozek M (2004) Computational homology. Springer-Verlag, Berlin
Edelsbrunner H, Letscher D, Zomorodian A (2001) Topological persistence and simplification. Discret Comput Geom 28:511–533
Zomorodian A, Carlsson G (2005) Computing persistent homology. Discret Comput Geom 33:249–274
Kasson PM, Zomorodian A, Park S, Singhal N, Guibas LJ, Pande VS (2007) Persistent voids a new structural metric for membrane fusion. Bioinformatics 23:1753–1759
Dabaghian Y, Mémoli F, Frank L, Carlsson G (2012) A topological paradigm for hippocampal spatial map formation using persistent homology. PLoS Comput Biol 8(8):e1002581
Gameiro M, Hiraoka Y, Izumi S, Kramar M, Mischaikow K, Nanda V (2014) Topological measurement of protein compressibility via persistence diagrams. Jpn J Ind Appl Math 32:1–17
Xia KL, Wei GW (2014) Persistent homology analysis of protein structure, flexibility and folding. Int J Numer Method Biomed Eng 30:814–844
Xia KL, Wei GW (2015) Persistent topology for cryo-EM data analysis. Int J Numer Method Biomed Eng 31:e02719
Xia KL, Feng X, Tong YY, Wei GW (2015) Persistent homology for the quantitative prediction of fullerene stability. J Comput Chem 36:408–422
Wang B, Wei GW (2016) Object-oriented persistent homology. J Comput Phys 305:276–299
Liu B, Wang B, Zhao R, Tong Y, Wei G-W (2017) Eses: software for e ulerian solvent excluded surface. J Comput Chem 38(7):446–466
Cang ZX, Mu L, Wu K, Opron K, Xia K, Wei G-W (2015) A topological approach to protein classification. Mol Based Math Biol 3:140–162
Cang ZX, Wei GW (2017) Analysis and prediction of protein folding energy changes upon mutation by element specific persistent homology. Bioinformatics 33:3549–3557
Wu K, Wei GW (2018) Quantitative toxicity prediction using topology based multitask deep neural networks. J Chem Inf Model 58:520–531
Wu K, Zhao Z, Wang R, Wei GW (2018) TopP-S: persistent homology-based multi-task deep neural networks for simultaneous predictions of partition coefficient and aqueous solubility. J Comput Chem 39:1444–1454
Hosoya H (1971) Topological index. a newly proposed quantity characterizing the topological nature of structural isomers of saturated hydrocarbons. Bull Chem Soc Jpn 44(9):2332–2339
Hansen PJ, Jurs PC (1988) Chemical applications of graph theory. Part i. Fundamentals and topological indices. J Chem Educ 65(7):574
Newman M (2010) Networks: an introduction. Oxford University Press, Oxford
Bavelas A (1950) Communication patterns in task-oriented groups. J Acoust Soc Am 22(6):725–730
Dekker A (2005) Conceptual distance in social network analysis. J Soc Struct 6:31
Bahar I, Atilgan AR, Erman B (1997) Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Fold Des 2:173–181
Yang LW, Chng CP (2008) Coarse-grained models reveal functional dynamics-I. Elastic network models-theories, comparisons and perspectives. Bioinf Biol Insights 2:25–45
Wei GW, Zhan M, Lai CH (2002) Tailoring wavelets for chaos control. Phys Rev Lett 89:284103
Go N, Noguti T, Nishikawa T (1983) Dynamics of a small globular protein in terms of low-frequency vibrational modes. Proc Natl Acad Sci USA 80:3696–3700
Tasumi M, Takenchi H, Ataka S, Dwidedi AM, Krimm S (1982) Normal vibrations of proteins: glucagon. Biopolymers 21:711–714
Brooks BR, Bruccoleri RE, Olafson BD, States D, Swaminathan S, Karplus M (1983) Charmm: a program for macromolecular energy, minimization, and dynamics calculations. J Comput Chem 4:187–217
Levitt M, Sander C, Stern PS (1985) Protein normal-mode dynamics: trypsin inhibitor, crambin, ribonuclease and lysozyme. J Mol Biol 181(3):423–447
Flory PJ (1976) Statistical thermodynamics of random networks. Proc R. Soc. Lond. A 351:351–378
Bahar I, Atilgan AR, Demirel MC, Erman B (1998) Vibrational dynamics of proteins: significance of slow and fast modes in relation to function and stability. Phys Rev Lett 80:2733–2736
Atilgan AR, Durrell SR, Jernigan RL, Demirel MC, Keskin O, Bahar I (2001) Anisotropy of fluctuation dynamics of proteins with an elastic network model. Biophys J 80:505–515
Hinsen K (1998) Analysis of domain motions by approximate normal mode calculations. Proteins 33:417–429
Tama F, Sanejouand YH (2001) Conformational change of proteins arising from normal mode calculations. Protein Eng 14:1–6
Cui Q, Bahar I (2010) Normal mode analysis: theory and applications to biological and chemical systems. Chapman and Hall, London
Balaban AT (1976) Chemical applications of graph theory. Academic Press, Cambridge
Trinajstic N (1983) Chemical graph theory. CRC Press, Boca Raton
Schultz HP (1989) Topological organic chemistry. 1. Graph theory and topological indices of alkanes. J Chem Inf Comput Sci 29(3):227–228
Foulds LR (2012) Graph theory applicatons. Springer, Berlin
Ozkanlar A, Clark AE (2014) Chemnetworks: a complex network analysis tool for chemical systems. J Comput Chem 35(6):495–505
Di Paola L, Giuliani A (2015) Protein contact network topology: a natural language for allostery. Curr Opin Struct Biol 31:43–48
Canutescu AA, Shelenkov AA, Dunbrack RL (2003) A graph-theory algorithm for rapid protein side-chain prediction. Protein Sci 12(9):2001–2014
Ryslik GA, Cheng Y, Cheung K-H, Modis Y, Zhao H (2014) A graph theoretic approach to utilizing protein structure to identify non-random somatic mutations. BMC Bioinform 15(1):86
Jacobs DJ, Rader AJ, Kuhn LA, Thorpe MF (2001) Protein flexibility predictions using graph theory. Proteins-Struct Funct Genet 44:150–165
Vishveshwara S, Brinda K, Kannan N (2002) Protein structure: insights from graph theory. J Theor Comput Chem 1(01):187–211
Wu Z, Ramsundar B, Feinberg EN, Gomes J, Geniesse C, Pappu AS, Leswing K, Pande V (2017) Moleculenet: A benchmark for molecular machine learning. arXiv preprint arXiv:1703.00564
Quan L, Lv Q, Zhang Y (2016) Strum: structure-based prediction of protein stability changes upon single-point mutation. Struct Bioinform (In press)
Pires DEV, Ascher DB, Blundell TL (2014) mcsm: predicting the effects of mutations in proteins using graph-based signatures. Struct Bioinform 30:335–342
Park JK, Jernigan R, Wu Z (2013) Coarse grained normal mode analysis vs. refined gaussian network model for protein residue-level structural fluctuations. Bull Math Biol 75:124–160
Bramer D, Wei GW (2018) Weighted multiscale colored graphs for protein flexibility and rigidity analysis. J Chem Phys 148:054103
Goodfellow I, Pouget-Abadie J, Mirza M, Xu B, Warde-Farley D, Ozair S, Courville A, Bengio Y (2014) Generative adversarial nets. In: Mozer MC, Jordan MI, Petsche T (eds) Advances in neural information processing systems. MIT Press, Cambridge, pp 2672–2680
Xia KL, Opron K, Wei GW (2013) Multiscale multiphysics and multidomain models—flexibility and rigidity. J Chem Phys 139:194109
Opron K, Xia KL, Wei GW (2014) Fast and anisotropic flexibility-rigidity index for protein flexibility and fluctuation analysis. J Chem Phys 140:234105
Nguyen DD, Xia KL, Wei GW (2016) Generalized flexibility-rigidity index. J Chem Phys 144:234106
Wei GW (2000) Wavelets generated by using discrete singular convolution kernels. J Phys A 33:8577–8596
Soldea O, Elber G, Rivlin E (2006) Global segmentation and curvature analysis of volumetric data sets using trivariate b-spline functions. IEEE Trans PAMI 28(2):265–278
Edelsbrunner H (1992) Weighted alpha shapes. Technical Report. University of Illinois, Champaign
Srivastava N, Hinton GE, Krizhevsky A, Sutskever I, Salakhutdinov R (2014) Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res 15(1):1929–1958
Arjovsky M, Chintala S, Bottou L (2017) Wasserstein generative adversarial networks. In: International conference on machine learning, pp 214–223
Mirza M, Osindero S (2014) Conditional generative adversarial nets. arXiv preprint arXiv:1411.1784
Acknowledgements
This work was supported in part by NSF Grants DMS-1721024, DMS-1761320, and IIS1900473 and NIH Grant GM126189. DDN and GWW are also funded by Bristol-Myers Squibb and Pfizer.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations/
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nguyen, D.D., Gao, K., Wang, M. et al. MathDL: mathematical deep learning for D3R Grand Challenge 4. J Comput Aided Mol Des 34, 131–147 (2020). https://doi.org/10.1007/s10822-019-00237-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-019-00237-5