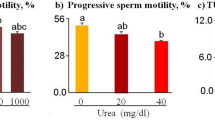
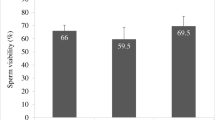
Abstract
Purpose
Motility of spermatozoa helps not only in planning the type of infertility treatment but also directly reflects the success rate in assisted reproductive technology (ART). Previously, biotin, a water-soluble vitamin, has been shown to increase the motility and longevity of cryopreserved human spermatozoa. The present study was designed to understand the molecular basis of the beneficial effects of presence of biotin in sperm wash medium on early embryo development.
Methods
The effect biotin supplementation to sperm wash medium on the sperm parameters were assessed in swim-up fraction of normozoospermic and asthenozoospermic ejaculates collected from infertile men. Fertilization and early embryo development was studied using Swiss albino mice.
Results
Even though both biotin and pentoxifylline (PTX) enhanced the motility of spermatozoa from normozoospermic and asthenozoospermic samples, biotin group exhibited higher in vitro survival. Using mouse model, we observed that presence of biotin or PTX in sperm wash medium improved the fertilization rate and blastocyst rate compared to control. Blastocysts from these groups had significantly higher total cell number (P < 0.01) and lower apoptotic index. In silico target prediction revealed that GTPase HRas (HRas), tyrosine-protein phosphatase nonreceptor type 1 (PTP1B), and glucokinase are the probable targets for biotin. Solution-state Nuclear Magnetic Resonance (NMR) studies confirmed that biotin interacts both with human HRas and PTP1B.
Conclusion
Our results indicate that presence of biotin in sperm wash medium can improve the fertilization potential and preimplantation embryo development and can be considered as a safe alternate to PTX.




Similar content being viewed by others
References
Irvine DS. Computer assisted semen analysis systems: sperm motility assessment. Hum Reprod. 1995;1:53–9.
Shulman A, Hauser R, Lipitz S, Frenkel Y, Dor J, Bider D, et al. Sperm motility is a major determinant of pregnancy outcome following intrauterine insemination. J Assist Reprod Genet. 1998;15:381–5.
Donnelly ET, Lewis SE, McNally JA, Thompson W. In vitro fertilization and pregnancy rates: the influence of sperm motility and morphology on IVF outcome. Fertil Steril. 1998;70:305–14.
Nagy ZP, Joris H, Verheyen G, Tournaye H, Devroey P, Van Steirteghem AC, Correlation between motility of testicular spermatozoa, testicular morphology and the outcome of intracytoplasmic sperm injection. Hum Reprod 1998; 13:890–895.
Oehninger S, Sueldo C, Lanzendorf S, Mahony M, Burkman LJ, Alexander NJ, et al. A sequential analysis of the effect of progesterone on specific sperm functions crucial to fertilization in vitro in infertile patients. Hum Reprod. 1994;9:1322–7.
Yanagimachi R. In vitro capacitation of hamster spermatozoa by follicular fluid. J Reprod Fertil. 1969;18:275–86.
Kalthur G, Kumar P, Adiga SK. Enhancement in motility of sperm co-incubated with cumulus oocyte complex (COC) in vitro. Eur J Obstet Gynecol Reprod Biol. 2009;145:167–71.
Schill WB. Caffeine- and kallikrein-induced stimulation of human sperm motility: a comparative study. Andrologia. 1975;7:229–36.
Yovich JM, Edirisinghe WR, Cummins JM, Yovich JL. Preliminary results using pentoxifylline in a pronuclear stage tubal transfer (PROST) program for severe male factor infertility. Fertil Steril. 1988;50:179–81.
Yovich JM, Edirisinghe WR, Cummins JM, Yovich JL. Influence of pentoxifylline in severe male factor infertility. Fertil Steril. 1990;53:715–22.
Tasdemir M, Tasdemir I, Kodama H, Tanaka T. Pentoxifylline-enhanced acrosome reaction correlates with fertilization in vitro. Hum Reprod. 1993;8:2102–7.
Tesarik J, Mandoza C. Sperm treatment with pentoxifylline improves the fertilizing ability in patients with acrosome reaction insufficiency. Fertil Steril. 1993;60:141–8.
Tournaye H, Janssens R, Camus M, Staessen C, Devroey P, Van Steirteghem A. Pentoxifylline is not useful in enhancing sperm function in cases with previous in vitro fertilization failure. Fertil Steril 1993; 59:210–215.
Tournaye H, Janssens R, Devroey P, Van Steirteghem A. The influence of pentoxifylline on motility and viability of spermatozoa from normozoospermic semen samples. Int J Androl 1994; 17:1–8.
Dimitriadou F, Rizos D, Mantzavinos T, Arvaniti K, Voutsina K, Prapa A, et al. The effect of pentoxifylline on sperm motility, oocyte fertilization, embryo quality, and pregnancy outcome in an in vitro fertilization program. Fertil Steril. 1995;63:880–6.
Tesarik J, Mendoza C, Carreras A. Effects of phopshodiesterase inhibitors caffeine and pentoxifylline on spontaneous and stimulus-induced acrosome reaction in human sperm. Fertil Steril. 1992;58:1185–90.
Tournaye H, Janssens R, Verheyen G, Devroey P, Van Steirteghem A. An indiscriminate use of pentoxifylline does not improve in-vitro fertilization in poor fertilizers. Hum Reprod 1994; 9:1289–1292.
Lacham-Kaplan O, Trounson AO. Embryo development capacity of oocytes fertilized by immature sperm and sperm treated with motility stimulants. Reprod Fertil Dev. 1994;6:113–6.
Scott L, Smith S. Human sperm motility-enhancing agents have detrimental effects on mouse oocytes and embryos. Fertil Steril. 1995;63:166–75.
Mock DM, Mock NI, Stewart CW, LaBorde JB, Hansen DK. Marginal biotin deficiency is teratogenic in ICR mice. J Nutr. 2003;133:2519–25.
Watanabe T, Dakshinamurti K, Persaud TV. Biotin influences palatal development of mouse embryos in organ culture. J Nutr. 1995;125:2114–21.
Kalthur G, Salian SR, Keyvanifard F, Sreedharan S, Thomas JS, Kumar P, et al. Supplementation of biotin to sperm preparation medium increases the motility and longevity in cryopreserved human spermatozoa. J Assist Reprod Genet. 2012;29:631–5.
Liu DY, Baker HW. Calcium ionophore-induced acrosome reaction correlates with fertilization rates in vitro in patients with teratozoospermic semen. Hum Reprod. 1998;13:905–10.
Kotdawala AP, Kumar S, Salian SR, Thankachan P, Govindraj K, Kumar P, et al. Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J Assist Reprod Genet. 2012;29:1447–53.
Kalthur G, Salian SR, Nair R, Mathew J, Adiga SK, Kalthur SG, et al. Distribution pattern of cytoplasmic organelles, spindle integrity, oxidative stress, octamer-binding transcription factor 4 (Oct4) expression and developmental potential of oocytes following multiple superovulation. Reprod Fertil Dev. 2016;28:2027–38.
Fielding L. NMR methods for the determination of protein-ligand dissociation constants. Curr Top Med Chem. 2003;3:39–53.
Ludwig C, Guenther UL. Ligand based NMR methods for drug delivery. Front Biosci. 2009;14:4565–74.
Cala O, Guillière F, Krimm I. NMR-based analysis of protein–ligand interactions. Anal Bioanal Chem. 2014;406:943–56.
Bakkialakshmi S, Chandrakala D. A spectroscopic investigations of anticancer drugs binding to bovine serum albumin. Spectrochim Acta Mol Biomol Spectrosc. 2012;88:2–9.
Tesarik J, Thebault A, Testart J. Effect of pentoxifylline on sperm movement characteristics in normozoospermic and asthenozoospermic specimens. Hum Reprod. 1992;7:1257–63.
Coccia ME, Becattini C, Criscuoli L, Fuzzi B, Scarselli G. A sperm survival test and in-vitro fertilization outcome in the presence of male factor infertility. Hum Reprod. 1997;12:1969–73.
Tournaye H, Van der Linden M, Van den Abbeel E, Devroey P, Van Steirteghem A. The effect of pentoxifylline on mouse in-vitro fertilization and early embryonic development. Hum Reprod 1994; 9:1903–1908.
Calogero AE, Fishel S, Hall J, Ferrara E, Vicari E, Green S, et al. Correlation between intracellular cAMP content, kinematic parameters and hyperactivation of human spermatozoa after incubation with pentoxifylline. Hum Reprod. 1998;13:911–5.
Jayaprakash D, Kumar KS, Shivaji S, Seshagiri PB. Pentoxifylline induces hyperactivation and acrosome reaction in spermatozoa of golden hamsters: changes in motility kinematics. Hum Reprod. 1997;12:2192–9.
Rupasri R, Jayaprakash D, Peter AT, Sreenivasa MS, Kumar M, Seshagiri PB. Pentoxifylline improves sperm capacitation and in vitro fertilization of oocytes in the golden hamster. Theriogenol. 1995;44:553–62.
Zempleni J, Teixeira DC, Kuroishi T, Cordonier EL, Baier S. Biotin requirements for DNA damage prevention. Mutat Res. 2012;733:58–60.
Dakshinamurti K. Biotin—a regulator of gene expression. J Nutr Biochem. 2005;16:419–23.
Barbacid M. Ras genes. Annu Rev Biochem. 1987;56:779–827.
Cantley LC, Auger KR, Carpenter C, Duckworth B, Graziani A, Kapeller R, et al. Oncogenes and signal transduction. Cell. 1991;64:281–302.
Naz RK, Ahmad K, Kaplan P. Expression and function of ras proto-oncogene proteins in human sperm cells. J Cell Sci. 1992;102:487–94.
Shi L, Zhang Q, Xu B, Jiang X, Dia Y, Zhang CY, et al. Sustained high protein-tyrosine phosphatase 1B activity in the sperm of obese males impairs the sperm acrosome reaction. J Biol Chem. 2014;289:8432–41.
Ruete MC, Lucchesi O, Bustos MA, Tomes CN. Epac, Rap and Rab3 act in concert to mobilize calcium from sperm’s acrosome during exocytosis. Cell Commun Signal. 2014;12:43.
Fernandez-Novell JM, Ballester J, Medrano A, Otaegui PJ, Guinovart JJ, Rodriguez-Hil JE. The presence of a high-Km hexokinase activity in dog, but not in boar, sperm. FEBS Lett. 2004;570:211–6.
Funding
The financial assistance from Indian Council of Medical Research (ICMR) is thankfully recognized (ICMR/5/10/10/2009-RHN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Study was approved by Institutional Ethics Committee of Kasturba Medical College, Manipal Academy of Higher Education, Manipal (IEC 087/2010), and a written informed consent was taken from subjects willing to provide their semen samples for the study. All the methods were performed in accordance with the institutional guidelines and Helsinki declaration. The study was approved by Institutional Animal Ethics Committee of Kasturba Medical College, Manipal (IAEC/KMC/40/2012). Animal care and handling were conducted according to the institutional guidelines for animal experimentation.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 182 kb)
Rights and permissions
About this article
Cite this article
Salian, S.R., Nayak, G., Kumari, S. et al. Supplementation of biotin to sperm preparation medium enhances fertilizing ability of spermatozoa and improves preimplantation embryo development. J Assist Reprod Genet 36, 255–266 (2019). https://doi.org/10.1007/s10815-018-1323-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-018-1323-1