Abstract
Purpose
Spermatozoa maturation, a process required for spermatozoa to acquire progressive motility and the ability to fertilize ova, primarily occurs in the caput and corpus of the epididymis. Despite considerable efforts, the factor(s) promoting epididymal sperm maturation remains unclear. Recently, WNT signaling has been implicated in epididymal sperm maturation.
Methods
To further investigate WNT signaling function in epididymal sperm maturation, we generated Wntless conditional knockout mice (Wls cKO), Wls flox/flox ; Lcn5-Cre.
Results
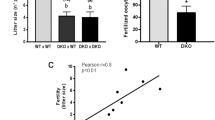
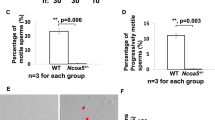
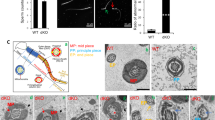
In these mice, WNTLESS (WLS), a conserved membrane protein required for all WNT protein secretion, was specifically disrupted in the principal cells of the caput epididymidis. Immunoblot analysis showed that WLS was significantly reduced in the caput epididymidis of Wls cKO mice. In the caput epididymidis of Wls cKO mice, WNT 10A and WNT 2b, which are typically secreted by the principal cells of the caput epididymis, were not secreted. Interestingly, sperm motility analysis showed that the WLS deficiency in the caput epididymidis had no effect on sperm motility. Moreover, fertility tests showed that Wls cKO male mice had normal fertility.
Conclusion
These results indicate that the disruption of WLS in principal cells of the caput epididymidis inhibits WNT protein secretion but has no effect on sperm motility and male fertility, suggesting that WNT signaling in the caput epididymidis may be dispensable for epididymal sperm maturation in mice.




Similar content being viewed by others
References
Cornwall GA. New insights into epididymal biology and function. Hum Reprod Update. 2009;15:213–27.
Sipilä P, Jalkanen J, Huhtaniemi I, Poutanen M. Novel epididymal proteins as targets for the development of posttesticular male contraception. Reproduction. 2009;137:379–89.
Turner TT. De Graaf’s thread: the human epididymis. J Androl. 2008;29:237–50.
Sullivan R, Saez F, Girouard J, Frenette G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cell Mol Dis. 2005;35:1–10.
Cooper T. Interactions between epididymal secretions and spermatozoa. J Reprod Fertil Suppl. 1997;53:119–36.
Hermo L, Oka R, Morales CR. Secretion and endocytosis in the male reproductive tract: a role in sperm maturation. Int Rev Cytol. 1994;154:105–89.
Dacheux J-L, Dacheux F. New insights into epididymal function in relation to sperm maturation. Reproduction. 2014;147:R27–42.
Koch S, Acebron SP, Herbst J, Hatiboglu G, Niehrs C. Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell. 2015;163:1225–36.
Zhang J-S, Liu Q, Li Y-M, Hall SH, French FS, Zhang Y-L. Genome-wide profiling of segmental-regulated transcriptomes in human epididymis using oligo microarray. Mol Cell Endocrinol. 2006;250:169–77.
Dubé E, Chan PT, Hermo L, Cyr DG. Gene expression profiling and its relevance to the blood-epididymal barrier in the human epididymis 1. Biol Reprod. 2007;76:1034–44.
Thimon V, Koukoui O, Calvo E, Sullivan R. Region-specific gene expression profiling along the human epididymis. Mol Hum Reprod. 2007;13:691–704.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810.
Clevers H. Wnt/β-catenin signaling in development and disease. Cell. 2006;127:469–80.
Chen SR, Tang J, Cheng J, Hao X, Wang Y, Wang X, et al. Does murine spermatogenesis require WNT signalling? A lesson from Gpr177 conditional knockout mouse models. Cell Death Dis. 2016;7:e2281.
Boyer A, Yeh JR, Zhang X, Paquet M, Gaudin A, Nagano MC, et al. CTNNB1 signaling in sertoli cells downregulates spermatogonial stem cell activity via WNT4. PLoS One. 2012;7:e29764.
Takase HM, Nusse R. Paracrine Wnt/β-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc Natl Acad Sci. 2016;113:E1489–E97.
Bernard P, Harley VR. Wnt4 action in gonadal development and sex determination. Int J Biochem Cell Biol. 2007;39:31–43.
Yeh JR, Zhang X, Nagano MC. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J Cell Sci. 2011;124:2357–66.
Jeays-Ward K, Dandonneau M, Swain A. Wnt4 is required for proper male as well as female sexual development. Dev Biol. 2004;276:431–40.
Wang K, Li N, Yeung C-H, Cooper TG, Liu X-X, Liu J, et al. Comparison of gene expression of the oncogenic Wnt/β-catenin signaling pathway components in the mouse and human epididymis. Asian J Androl. 2015;17:1006.
Deshpande SN, Vijayakumar G, Rao AJ. Oestrogenic regulation and differential expression of WNT4 in the bonnet monkey and rodent epididymis. Reprod BioMed Online. 2009;18:555–61.
Bänziger C, Soldini D, Schütt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–22.
Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–33.
Fu J, Jiang M, Mirando AJ, Yu H-MI, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proc Natl Acad Sci. 2009;106:18598–603.
Coombs GS, Yu J, Canning CA, Veltri CA, Covey TM, Cheong JK, et al. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123:3357–67.
Das S, Yu S, Sakamori R, Stypulkowski E, Gao N. Wntless in Wnt secretion: molecular, cellular and genetic aspects. Front Biol. 2012;7:587.
Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 2011;240:365–71.
Xie S, Xu J, Ma W, Liu Q, Han J, Yao G, et al. Lcn5 promoter directs the region-specific expression of cre recombinase in caput epididymidis of transgenic mice. Biol Reprod. 2013;88:71.
Jimenez T, Sánchez G, Wertheimer E, Blanco G. Activity of the Na, K-ATPase α4 isoform is important for membrane potential, intracellular Ca2+, and pH to maintain motility in rat spermatozoa. Reproduction. 2010;139:835–45.
Liu C, Song Z, Wang L, Yu H, Liu W, Shang Y, et al. Sirt1 regulates acrosome biogenesis by modulating autophagic flux during spermiogenesis in mice. Development. 2017;144:441–51.
Cheng JM, Li J, Tang J-X, Chen S-R, Deng S-L, Jin C, et al. Elevated intracellular pH appears in aged oocytes and causes oocyte aneuploidy associated with the loss of cohesion in mice. Cell Cycle. 2016;15:2454–63.
Dacheux JL, Castella S, Gatti JL, Dacheux F. Epididymal cell secretory activities and the role of proteins in boar sperm maturation. Theriogenology. 2005;63:319–41.
Xu J, Yao G, Ru Y, Xie S. Expression of tamoxifen-inducible CRE recombinase in Lcn5-CreERT2 transgenic mouse caput epididymis. Mol Reprod Dev. 2016;3:257–64.
Yu X, Suzuki K, Wang Y, Gupta A, Jin R, Orgebin-Crist MC, et al. The role of forkhead box A2 to restrict androgen-regulated gene expression of lipocalin 5 in the mouse epididymis. Mol Endocrinol. 2006;20:2418–31.
Zecca M, Basler K, Struhl G. Direct and long-range action of a wingless morphogen gradient. Cell. 1996;87:833–44.
Lawrence PA, Struhl G. Morphogens, compartments, and pattern: lessons from drosophila? Cell. 1996;85:951–61.
O'Hara L, Welsh M, Saunders PTK, Smith LB. Androgen receptor expression in the caput epididymal epithelium is essential for development of the initial segment and epididymal spermatozoa transit. Endocrinology. 2011;152:718–29.
Björkgren I, Saastamoinen L, Krutskikh A, Huhtaniemi I, Poutanen M, Sipilä P. Dicer1 ablation in the mouse epididymis causes dedifferentiation of the epithelium and imbalance in sex steroid signaling. PLoS One. 2012;7:e38457.
Krutskikh A, Poliandri A, Cabrera-Sharp V, Dacheux JL, Poutanen M, Huhtaniemi I. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. 2012;26:4198–209.
Acknowledgements
The authors would like to thank Xing-Xu Huang and Yong-Lian Zhang for kindly providing the Lcn5-Cre transgenic mice.
Funding
This work was supported by grants from the Major Research Plan “973” Project (2012CB944702), the Natural Science Foundation of China (31501953, 31501161, 31471352, 31471400, 81270662, and 31171380), and the Academician Workstation Support (Shenyang, Changsha and Shandong).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were performed in accordance with the protocols approved by the Institutional Animal Care and Use Committee at the Institute of Zoology (IOZ), Chinese Academy of Sciences (CAS).
Rights and permissions
About this article
Cite this article
Cheng, JM., Tang, JX., Li, J. et al. Role of WNT signaling in epididymal sperm maturation. J Assist Reprod Genet 35, 229–236 (2018). https://doi.org/10.1007/s10815-017-1066-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-1066-4