Abstract
Marine microalgae often live in a fluctuating environment including a decrease in salinity caused by global warming induced sea ice melting and freshwater inflows. Under conditions of fluctuating salinity, microalgae have evolved a variety of survival mechanisms such as lipid accumulation and remodeling. The purpose of this study was to investigated the membrane lipid remodeling of the marine green microalga Dunaliella tertiolecta as a short-term acclimation mechanism in response to hyposalinity (20 and 3 PSU) with respect to growth at optimal salinity (38 PSU). We identified 34 lipid species belonging to seven polar lipid classes. Dunaliella tertiolecta accumulates cell lipids and remodels polar lipid classes and their fatty acids composition as response to hypoosmotic stress at 3 PSU. We found that the unsaturation of most polar lipids decreases overall, indicating decreased membrane fluidity and altered permeability, whereas shortening the length of fatty acids of polar lipids is not one of the strategies of D. tertiolecta to cope with the decrease in salinity. Increase in relative content (%) and unsaturation of monogalactosyldiacylglycerols (MGDG) and decrease in relative content (%) and unsaturation of phosphatidylglycerols (PG), suggesting changes in photosynthetic membranes of thylakoids at 20 and 3 PSU. At a very low salinity of 3 PSU, the relative content (%) of phosphatidylinositols (PI) increases, suggesting increased lipid trafficking and signaling in the cells. These changes are statistically significant and we hypothesize that D. tertiolecta is genetically adapted to withstand large salinity fluctuations through polar lipid composition.
Similar content being viewed by others
Introduction
Eukaryotic phytoplankton (microalgae) are photosynthetic organisms and the most important primary producers in aquatic ecosystems, responsible for photosynthetic fixation of about 50% of atmospheric carbon (Falkowski and Raven 2007). They possess remarkable plasticity and adaptability to abiotic stresses and can grow in a wide range of habitats (Shetty et al. 2019). Microalgae synthesize various fatty acids, but the most important are long chain polyunsaturated fatty acids (PUFA), which are necessary for healthy and balanced growth of higher trophic levels that have limited capacity to synthesize these fatty acids (Arts et al. 2001).
Ocean salinity has changed in the last 50 years; the Pacific and Southern Ocean are becoming fresher and the Atlantic and Indian Oceans are becoming saltier due to global climate change (Cheng et al. 2020). Salinity is highly variable in coastal regions, especially in estuaries and rock pools (Kirst 1990). Salinity fluctuation is one of the major abiotic stressors that induces an oxidative stress response in algae, affects external water potential, and disturbs turgor pressure, ion distribution and organic solutes in the cell, and inhibits cell division and growth rate (Richmond 1986; Kirst 1990; Mishra et al. 2008; Shetty et al. 2019). Microalgae respond and adapt differently to salt stress (Kirst 1990), including changes in photosynthesis, photorespiration, osmotic adjustment and compartmentalization, and amino acid and carbohydrate synthesis (Kirst 1990; Tammam et al. 2011; Neelam and Subramanyam 2013).
Lipids (phospholipids, glycolipids and betaine lipids) in microalgae cells are important molecules that play vital roles in cell structure, signaling and trafficking and are essential for tolerance and acclimation to overcome stressful environmental conditions (Harwood and Guschina 2009; Du and Benning 2016). A major component of plasma membrane lipids in microalgae are the phospholipids phosphatidylcholines (PC), phosphatidylethanolamines (PE) and PG. Glycolipids play important roles in forming thylakoid lipid bilayers and protein assembly in photosynthetic complexes (Kobayashi 2016). Phosphatidylglycerol, the only phospholipid found in thylakoids, is essential for photosynthetic electron transport, the development of chloroplasts, and tolerance to chilling (Wada and Murata 2007). The phospholipid PI serves as a precursor for synthesizing other phospholipids (Harwood and Guschina 2009) and plays an important regulatory role in the cell physiology (Furt et al. 2011). For many years betaine lipids in microalgae were thought to serve as an adaptive strategy to cope with phosphate deficiency, but in certain algal species betaine lipids play a role other than of a mere substitute for phospholipids, due to their much greater unsaturation than in phospholipids (Murakami et al. 2018 and references therein). Regulation of lipid biosynthesis is one of the most important strategies in resistance physiology to salinity stress in microalgae (Guschina and Harwood 2006).
The genus Dunaliella is a unicellular photoautotrophic green microalgae with more than 20 marine and halophilic species (Borowitzka and Siva 2007) found in a wide range of waters, from marine areas to salinity variable brackish lagoons and estuaries (Borowitzka and Brown 1974; Gilmour et al. 1984; Ginzburg 1988; Borowitzka 2018a). Dunaliella also inhabits hypersaline environments, where it is responsible for most of the primary production (Oren 2005). In Dunaliella the cell wall is replaced by a thin, elastic plasma membrane and a glycocalyx surface coat that makes the cells morphologically more flexible (rapid changes in cell volume) to changes in salinity (Hamburger 1905; Oliveira et al. 1980; Svetličić et al. 2001; Oren 2005; Novosel and Ivošević DeNardis 2021). Under stressful environmental conditions, the cell can accumulate various economically valuable organic compounds such as vitamins, proteins and carotenoids (Ghoshal et al. 2002; Hadi et al. 2008; Borowitzka 2013).
Dunaliella tertiolecta is frequently exposed to hypoosmotic stress in its usual habitat (Gilmour et al. 1984). To date, there are only two papers on D. tertiolecta dealing with individual lipid classes and their remodeling during growth under stress conditions (light, temperature and nutrient N-deplete) (Kim et al. 2013, 2015). Several published papers on D. tertiolecta growth at different salinities focused on the content and composition of free fatty acid, total lipid content, glycerol synthesis/degradation, synthesis of antioxidant enzymes and β-carotene (Borowitzka and Brown 1974; Pick 2002; Jahnke and White 2003; Takagi et al. 2006; Tammam et al. 2011; Liang et al. 2017; Rizwan et al. 2020). The response of D. tertiolecta to hyposalinity includes an increase in cell size, a decrease in Fv/Fm (maximal photochemical efficienct of PS II) a decrease in ascorbate content, and an increase in the pools of α-tocopherol and total glutathione (Jahnke and White 2003). However, there is no report on individual lipid classes and lipid remodeling during growth of D. tertiolecta in hyposaline environment. Lipids play an essential biological role in microalgae cells as a defense response to stress conditions. Comprehension of the lipid remodeling will lead to a better understanding of microalgae acclimation/adaptation mechanisms to changes in salinity. Here, we investigate the acclimation of the marine green microalga D. tertiolecta to salinity decrease by analyzing changes in polar lipid composition. To elucidate lipid remodeling, lipidomics analysis based on hydrophilic interaction liquid chromatography coupled with electrospray ionization mass spectrometry (HILIC-ESI–MS) was used to identify and quantify lipid classes and their fatty acid composition. We demonstrate how D. tertiolecta remodels lipid content and fatty acids composition under hyposaline conditions. The statistically significant polar lipid remodeling is attributed to the fact that D. tertiolecta has already genetically adapted to a wide range of salinities through the composition of membrane lipids.
Materials and methods
Algal culture and growing conditions
Dunaliella tertiolecta (Chlorophyceae, CCMP 1320, Culture Collection Bigelow, Laboratory for Ocean Sciences, Bigelow, MN, USA) was used. Prior to the experiments, the microalga was grown (for two weeks) in seawater (salinity of 38 PSU (Practical Salinity Unit)) enriched with f/2 medium (Guillard and Ryther 1962; Guillard 1975) in 200 mL sterile VWR tissue culture flasks (VWR, USA). The different salinities of the enriched f/2 culture medium were prepared as follows: 1000 mL seawater (38 PSU), 480 mL seawater and 520 mL deionised water (20 PSU), and 80 mL seawater and 920 mL deionized water (3 PSU). The culture media were inoculated with 4 × 104 cells mL−1. Cultures were gently stirred twice a day manually and counted every 2 to 3 days using Fuchs-Rosenthal counting chambers. All experiments were performed at 18 °C in a thermostatic chamber (Inkolab, Croatia) with a light/dark cycle of 12/12 h under an illumination of 72 µmol photons m−2 s−1. Growth was terminated when the cells reached the third day of stationary phase. All experiments were performed in triplicate.
Chemicals
Lipid standards MGDG, digalactosyldiacylglycerols (DGDG), sulfoquinovosyldiacylglycerols (SQDG), PG, PC, PE, PI, phosphatidylserine (PS), phosphatidic acids (PA) and diacylglyceryl-N,N,N-trimethylhomoserines (DGTS) were purchased from Avanti Polar Lipids (Alabaster, AL, USA), while all other lipid standards were purchased from Sigma-Aldrich (Sigma-Aldrich Corporation, Saint Louis, MO, USA). The internal standards (Fmoc-glycine) and ammonium acetate were purchased from EMD Millipore (Merck Millipore, Burlington, MA, USA). Formic acid was purchased from Fluka (Honeywell International Inc, Charlotte, NC, USA). All solvents (acetonitrile, water, dichloromethane, methanol and isopropanol) were purchased from J.T.Baker (Phillipsburg, NJ, USA) and were HPLC-grade.
Lipid extraction
For lipid analysis, 100 mL of the culture was filtered through a 0.7 μm Whatman GF/F filter (pre-burned at 450 ºC for 5 h). The filters were then stored at – 80 °C prior to analysis. The lipid fraction was extracted according to the modified Bligh and Dyer method (Bligh and Dyer 1959). Extracts were evaporated to dryness under nitrogen and stored at – 20 °C until analysis. For HILIC-ESI–MS, dry lipid extracts were dissolved in dichloromethane:methanol (4:1 (v:v), 100 µL) and diluted with methanol:isopropanol (1:1 (v:v)) prior to analysis. An internal standard (Fmoc-glycine) was then added and samples were filtered through CHROMAFIL Xtra PTFE-20/13 filters (Macherey–Nagel, Germany). For thin-layer chromatographic analysis, dry lipid extracts were dissolved in 20 to 30 μL dichloromethane and an internal standard (n-nonadecanone) was added to each sample to estimate recovery.
Analysis of phospholipids, glycolipids and betaine lipids
Separation was performed with Waters Acquity HPLC system (Waters Corp, USA) equipped with a Hydrophilic Interaction Liquid Chromatography column (Acquity UPLC BEH HILIC 1.7 µm, 2.1 × 150 mm, Waters), using gradient elution. Mobile phase A was acetonitrile:water (95:5), and mobile phase B was water. Both phases contained 10 mM ammonium acetate and 0.1% formic acid. The gradient started at 100% A for 4 min, increased to 15% B over 8 min, and was held at 15% B for 1 min before returning to initial conditions after 1 min and held for 7 min. The flow rate was 0.3 mL min−1 and the injected sample volume was 1 µL. The temperature of the column and sample was 40 and 8 °C, respectively.
A quadrupole time-of-flight electrospray ionization mass spectrometer (QqToF Premier, Waters, USA) was used to identify and quantify lipids. Lipid analysis was performed in positive and negative ion modes. The optimal conditions were as follows: capillary voltage 3 kV; sampling cone voltage 20 V; source temperature 100 °C; desolvation temperature 300 °C; collision gas argon. Accurate mass was maintained by introducing a lock-spray interface of leucine enkephalin (556.2771 [M + H]+ or 554.2615 [M − H]−). Data were acquired in the mass range (m/z) of 50 – 1200 Da with a scan duration of 0.2 s. The MS/MS experiments were performed with the following collision energies: SGDG, DGDG, MGDG and PI 20 eV, DGTS, PA and PE 25 eV and PG and PC 30 eV. All lipids were detected in both ionization modes (positive and negative), but quantification of individual lipid species was performed in one ionization mode, while the other was used as a conformation. In the negative mode, MGDG and PG species were analyzed as [M + HCOO]− and [M-H]−, respectively. The remaining lipid classes were analyzed in positive mode; SQDG, DGDG and PI as [M + NH4]+ and PC and DGTS as [M + H]+. Instrument control and data analysis were performed using MassLynx 4.1 software (Waters). The concentration of each lipid was calculated from a calibration curve of known concentrations of the corresponding standards.
Lipid species are represented by the abbreviation of the lipid class (e.g. MGDG), while fatty acids are represented as x1:y1/x2:y2, where x1 and x2 are the number of carbon atoms in the first and second fatty acyl chain, and y1 and y2 are the number of double bonds in the first and second fatty acyl chain (e.g. MGDG 18:2/16:3).
Analysis of total lipids
Total cellular lipids were quantified by thin-layer chromatography coupled with flame-ionisation detection (Iatroscan MK-VI, Iatron, SES GmbH—Analytical Systems, Germany). The Iatroscan was operated at a hydrogen flow rate of 160 mL min−1 and an air flow rate of 2000 mL min−1. Total lipid content was calculated as the sum of the ten lipid classes detected (MGDG, SQDG, DGDG, PG, PE, PC, pigments, sterols, steryl esters and triacylglycerols). The detailed procedure is described in Gašparović et al. (2015, 2017).
Data analysis
The growth rate was calculated using the following equation:
where Nm and No are the algal concentrations at the end (day tm) and beginning (day to) of the exponential growth phase, and t is the time in days (Thompson et al. 1992).
Data are expressed as mean ± SD of three parallel measurements (three experiments for each salinity). A one-way ANOVA analysis (Microsoft Excel 2010 software) was used to examine the effect of salinity on lipid remodeling. A p-value < 0.05 is considered significant.
Results
Dunaliella tertiolecta growth
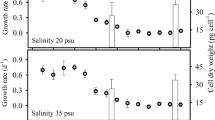
Growth curves of D. tertiolecta determined at salinities of 38, 20 and 3 PSU are shown in Fig. 1. A decrease in salinity has a significant effect on the growth of D. tertiolecta (p < 0.05). The highest cell density was observed at a salinity of 38 PSU (2.76 × 106 ± 2.34 × 105 cells mL−1), a 1.5-fold decrease in cell density was observed at salinity of 20 PSU (1.89 × 106 ± 1.53 × 105 cells mL−1), while the lowest cell density was observed at salinity of 3 PSU (2.73 × 105 ± 2.94 × 104 cells mL−1) (Fig. 1). The growth rate decreased (p < 0.05) from 0.217 ± 0.006 day−1 at 38 PSU to 0.181 ± 0.017 day−1 at 20 PSU and to 0.151 ± 0.026 day−1 at 3 PSU.
Lipidomic analysis
Lipidomic analysis of D. tertiolecta samples from the stationary growth phase identified 33 polar lipid species (two MGDG, three SQDG, six DGDG, four PG, one PI, five PC and 12 DGTS species). In addition, at optimal salinity (38 PSU), one additional MGDG species was identified.
The hyposaline environment affected lipid production (Fig. 2a, Table S2). The highest amount (not statistically significant) of polar lipids (2.07 ± 0.18 pg cell−1) was detected during growth at 3 PSU, while lower amounts of polar lipids were detected during growth at 20 and 38 PSU, 1.95 ± 0.08 and 1.77 ± 0.14 pg cell−1, respectively. Total cellular lipid content (polar lipids plus triacylglycerols and sterols) (1.73 ± 0.32 pg cell−1, 2.16 ± 0.37 pg cell−1, and 3.63 ± 0.43 pg cell−1 at 38, 20 and 3 PSU, respectively) statistically differs for studied salinities (p < 0.05). With decreasing salinity, the cellular content of total glycolipids (MGDG, SQDG and DGDG) and betaine lipids (DGTS) increased, whereas the content of total phospholipids (PG, PI and PC) decreased (Fig. 2a, Table S2) (p < 0.05). Significant changes are observed in the lipid classes MGDG and PG at 20 PSU with respect to 38 PSU (p < 0.05) (MGDG 36.5 ± 1.11% and 42.38 ± 1.78% and PG 17.74 ± 0.95% and 14.54 ± 0.62% at 38 and 20 PSU, respectively). The MGDG/DGDG ratio increased with salinity decrease (2.1, 2.4, and 2.5 for salinities of 38, 20, and 3 PSU, respectively).
Lipid profile of D. tertiolecta at 38, 20, and 3 PSU. Cell lipid content (pg cell−1) of glycolipids (GL), phospholipids (PL), betaine lipid DGTS (BET), total polar (Polar) and total cellular (CL) lipids (a) and composition of MGDG, SQDG, DGDG, PG, PI, PC and DGTS (expressed as percentage of total polar lipids—%) (b). Data are means ± SD (n = 3). Groups with different letters are statistically significantly different from each other (p < 0.05)
A statistically significant increase in MGDG, DGDG, PI, PC, and DGTS and a decrease in PG in total polar lipids were observed when salinity decreased from 38 to 3 PSU (p < 0.05; Fig. 2b, Table S3). The percentage of SQDG did not alter significantly in total polar lipid as salinity decrease.
The fatty acid composition of the identified lipid classes changed with salinity drop (Figs. 3–5, Table S4). The proportion of the most abundant MGDG 18:3/16:4 in total MGDG increased statistically significantly from 95.1 ± 0.5% at 38 PSU to 96.97 ± 0.37% at 20 PSU to 97.7 ± 0.3% at 3 PSU, while the proportion of MGDG 18:2/16:3 decreased from 4.70 ± 0.4% at 38 PSU to 3.03 ± 0.37% at 20 PSU and 2.3 ± 0.3% at 3 PSU (p < 0.05; Fig. 3a, Table S4).
The share of the most abundant SQDG 18:3/16:0 in the total SQDG decreased from 86.5 ± 0.7% at 38 PSU to 84.1 ± 1.7% at 3 PSU (not statistically significant), whereas SQDG 18:1/16:0 increased significantly (p < 0.05), while SQDG 16:0/16:0 remained statistically unchanged (Fig. 3b, Table S4). In parallel with the decrease in salinity from 38 to 3 PSU, the percentage of DGDG 18:3/16:3 decreased significantly (p < 0.05), while the relative proportions of DGDG 18:3/16:0, DGDG 18:2/16:0, DGDG 18:3/18:3 and DGDG 18:2/18:3 increased significantly (p < 0.05; Fig. 3c, Table S4) in total DGTS. The percentage of DGDG 18:1/16:0 in total DGDG remained unchanged.
The percentage of the most abundant PG 16:1/18:3 and PG 16:0/18:3 in the total PG decreased (p < 0.05) and the percentage of PG 16:0/18:2 increased (p < 0.05) when salinity decreased from 38 to 20 and 3 PSU, respectively (Fig. 4a, Table S4). The percentage of PG16:0/18:1 changed significantly between 38 and 20 PSU (p < 0.05). No remodeling occurred in PI, as only one PI18:1/16:0 species was identified at all three salinities. In the lipid class PC, no single species dominated at any salinity. The percentage of PC 18:3/18:2 in the total PC decreased between salinity of 38 and 3 PSU (Fig. 4b, Table S4). The percentage of PC 16:0/18:3 and PC 18:3/18:3 increased significantly (p < 0.05) in the total PC when salinity decreased from 38 to 20 PSU, and decreased significantly (p < 0.05) when salinity decreased from 20 to 3 PSU (Fig. 4b, Table S4). A small increase in PC 16:0/18:2 was observed when salinity decreased from 20 to 3 PSU (p < 0.05). The percentage of PC 18:3/18:1 decreased in the total PC when salinity decreased from 38 to 20 PSU and increased when salinity decreased from 20 to 3 PSU (p < 0.05; Fig. 4b, Table S4). Consequently, a small but significant (p < 0.05) decrease in total unsaturation was observed at 3 PSU compared to salinities 38 and 20 PSU (Fig. 6).
Betaine lipids were the most diverse lipid group, in which 12 different species were identified. When salinity decreased from 38 to 3 PSU, the relative amounts of DGTS 18:1/16:0, DGTS 18:3/18:4, DGTS 18:3/18:3, DGTS 18:3/18:2, and DGTS 18:3/18:1 decreased significantly (p < 0.05), whereas the relative amounts of DGTS 16:4/16:0, DGTS 18:2/16:0, DGTS 18:2/18:0, DGTS 18:3/16:0, and DGTS 18:3/18:0 significantly increased (p < 0.05) to the total DGTS (Fig. 5, Table S4). The most significant change was observed for DGTS 18:3/18:4, where there was a ~ 14-fold decrease (Fig. 5, Table S4) when D. tertiolecta grew under hyposaline conditions. The proportion of lipid species DGTS 16:3/16:0 and DGTS 18:4/16:0 did not differ significantly between 38 and 3 PSU, whereas it increased significantly when salinity decreased from 38 to 20 PSU (p < 0.05; Fig. 5, Table S4). A significant increase (p < 0.05) for DGDG 16:4/16:0 was observed for all salinities studied.
We identified only C16 and C18 fatty acids in all detected polar lipid species with different degrees of unsaturation (number of double bonds). A salinity drop resulted in changes in the distribution of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), PUFA, fatty acids C16, C18, C16:0, and C18:3 (Table 1.). All lipid classes were dominated by PUFA, with the exception of SQDG, in which similar proportions of SFA and PUFA were present. Hyposaline conditions (changes between 38 and 3 PSU) resulted in a decrease in the share of PUFA and a corresponding increase in the share of SFA for DGDG and DGTS (p < 0.05). Replacement of MUFA by SFA at 3 PSU was observed for PG (p < 0.05). Higher proportions of MUFA were observed in lipid classes SQDG and PC at 3 PSU. Significant changes (p < 0.05) in the proportion of MUFA were observed in the lipid classes PC and DGTS at all salinities studied.
The total unsaturation (sum of the double bonds of the two fatty acids) of the polar lipid classes is shown in Fig. 6. With the exception of SQDG, a statistically significant (p < 0.05) change in total unsaturation was observed for all other lipid classes at 3 PSU. Total unsaturation of MGDG was highest at salinity of 3 PSU, while it was lowest for lipid classes DGDG, PG, PC and DGTS. Significant (p < 0.05) changes in unsaturation were observed for lipid classes MGDG, PC and DGTS for salinity decrease from 38 to 20 PSU.
A change in fatty acid chain lengths with decreasing salinity was observed, i.e. replacement of C16 by C18 and vice versa (Table 1). In general, all lipids showed a similar distribution of C16 and C18, except PC, where C18 fatty acids dominated. A decrease to salinity of 3 PSU resulted in an elongation of fatty acid chains (C16 to C18) in DGDG and a shortening in DGTS (C18 to C16) (p < 0.05), whereas no significant changes were observed in the lipid classes MGDG, SQDG, PG and PC.
Omega-3 fatty acid, α-linolenic acid (18:3) type, was the most abundant PUFA in all lipid classes (Table 1). Its percentage decreased with decreasing salinity in all lipid classes, except in MGDG and DGDG, where it increased (p < 0.05). Significant changes (p < 0.05) were observed in the lipid classes MGDG, PG and PC for all salinities studied. Another abundant fatty acid was palmitic acid (16:0). Its share significantly (p < 0.05) increased under hyposaline conditions for DGDG, PG and DGTS lipid classes (Table 1) and significantly changed (p < 0.05) for PG at all salinities studied.
Discussion
Cell growth in response to salinity decrease
Dunaliella tertiolecta growth and reproduction are little affected when salinity decreases from an optimal salinity of 38 to 20 PSU, suggesting a very good physiological acclimation to such a significant salinity drop (Fig. 1, Table S1). The prolonged lag period (five days) of D. tertiolecta growth at 3 PSU compared to 38 and 20 PSU (two days), together with substantial retardation of growth and reproduction, suggest that the cells are under severe stress (Fig. 1, Table S1). Jahnke and White (2003) demonstrated that while different Dunaliella species can grow in a wide range of salinities (2.9 – 175 PSU) only D. tertiolecta can grow in salinity lower than 3 PSU, as we also observed here. A decrease in D. tertiolecta growth under hyposalinity was previously observed (Jahnke and White 2003; Rizwan et al. 2017). In addition, hyposalinity was found to lead to an increase in D. tertiolecta cell size, which had reduced Fv/Fm levels and a smaller ascorbate pool along with an increased pool of α-tocopherol and total glutathione (Jahnke and White 2003). It was also found that the rate of nutrient uptake by D. tertiolecta depends on the NaCl concentration (Hellebust 1985). All of these findings may explain here observed decrease in cell density after two days of growth at 3 PSU. Although, cell density decreases within two days, D. tertiolecta was able to adapt to hyposalinity stress, but with a longer lag phase and lower cell density after 16 days of growth compared to growth at 38 PSU. As previously noted, cell growth cannot resume after hyposalinity shock until the osmotic equilibrium of the cell is restored (Borowitzka 2018b).
Lipid accumulation in response to salinity decrease
As observed earlier, the influence of various stressful growth conditions (eg warming, nutrient depletion) leads to lipid accumulation in microalgae (Novak et al. 2019; Rizwan et al. 2020; Xiang et al. 2021). Growth of D. tertiolecta under hyposalinity also leads to accumulation of total cellular lipids (Fig. 2a, Table S2). This suggests that its complex intracellular biochemical processes are more focused on lipid synthesis under hyposaline conditions than under optimal salinity. Recently, reactive oxygen species formed in response to biotic or abiotic stressors were found to play a role in redirecting the cellular metabolism of Chlorella protothecoides (renamed Auxenochlorella protothecoides) to the accumulation of lipids instead of growth and storage of carbohydrates (Wang et al. 2016). The higher content of total cellular (pg cell−1) than total polar (pg cell−1) lipids (Fig. 2a, Table S2) is the result of the accumulation of non-polar triacylglycerols. Non-polar lipids in microalgae cells are energy-rich storage substances and are often synthesized under unfavorable environmental conditions, especially in response to nutrient deficiencies (Fields et al. 2014; Novak et al. 2019). The accumulation of non-polar lipids in hyposalinity could be a sign of cell enlargement and slowing of cell division in a hypoosmotic conditions, as has been proposed for Dunaliella salina (Yao et al. 2016).
Polar lipid classes remodeling in response to salinity decrease
Polar lipids play irreplaceable roles in microalgae cells. They are the major molecules that form cell and inner membranes and play an important role in cell signaling and trafficking (Harwood and Guschina 2009). Without the ability to remodel polar lipids under stressful conditions, microalgae cells cannot adapt/acclimatize and survive.
An increase in the cellular content of glycolipids (Fig. 2a, Table S2) and an increase in the relative abundance (% of total polar lipids) of especially nonionic MGDG and DGDG (Fig. 2b, Table S3) in parallel with a decrease in salinity, indicate thylakoid membranes remodeling. The observed increased ratio of non-bilayer to bilayer forming lipid, MGDG/DGDG, with decreasing salinity is likely related to the role of MGDG in photosynthesis. It is hypothesized that MGDG controls light harvesting of the light harvesting complex II by modulating the hydrostatic lateral membrane pressure sensed by the LHCII-bound peripheral pigments (Tietz et al. 2020). These simultaneously suggest changes in photosynthesis, as photosynthetic reactions depend mainly on the glycolipid-supported architecture of the photosynthetic machinery (Garab 2014). An increase in the relative abundance of bilayer-destabilizing glycolipids MGDG may adversely affect membrane stability and structural organization that consequently affect fluidity and permeability of the thylakoids (Pal et al. 2013). A significant increase in MGDG detected at 20 and 3 PSU (Fig. 2b, Table S3), suggests that MGDG remodeling is one of the first signals that cells are in an unfavorable hyposaline conditions. Acclimation of microalgae to stress may involve synthesis of new lipid species (Lu et al. 2012b), no fatty acid remodeling (Kim et al. 2013, 2015), as well as the absence of specific lipid species, as observed here for MGDG 18:3/18:3 at 20 and 3 PSU. Our data suggest that DGDG and especially MGDG are important in acclimation to salinity decrease.
The higher diversity of phospholipids (four PG, five PC and one PI) we identified compared to Kim et al. (2013) (two PG and one PI) may indicate that the two D. tertiolecta species are isolated from different regions.
The anionic lipids SQDG and PG may substitute each other to ensure a constant proportion of anionic lipids in thylakoids (Yu and Benning 2003). The significant decrease (60%) in the proportion of PG at 3 PSU in comparison to 38 PSU and no statistically significant changes in the proportion of SQDG in total polar lipids observed here (Fig. 2b, Table S3) indicate seriously compromised thylakoid membrane composition and probably function at 3 PSU. Liu et al. (2012) found significant inhibition of PSII efficiency in D. salina grown under hyposaline conditions. Phospholipid PG plays an essential role in photosynthetic activity (the maintenance and stability of the photosystem II) and cell growth (Lu et al. 2012a). Noh et al. (2020) considered that PG is involved in the cyanobacteria protection from high salinity, as a higher amount of PG was found in marine than in freshwater Synechocystis strains. The decrease in the proportion of PG in total lipids under hyposalinity (Fig. 2a, Table S1) indicate that growth at 20 and 3 PSU compromises PG synthesis. A reduced level of PG suggests impaired photosynthetic activity as reflected by the retardation of cell growth (Fig. 1) and growth rate at 20 and 3 PSU.
Our data suggest that extreme hyposalinity (3 PSU) causes replacement of PC (decrease in relative proportion) by DGTS (increase in relative proportion) (Fig. 2b, Table S3). Because both are zwitterionic lipids that have similar chemical and physical properties (Sato and Furuya 1985; Sato 1992), the assumption is reasonable. Betaine lipid DGTS (Eichenberger 1993) and PC (Moore 1982) are primarily localized in non-plastidial membranes. We may hypothesize that this replacement plays a role in altering plasma membrane permeability, as suggested for salt tolerance in plants (Mansour 2012). Betaine lipid DGTS is not present in all algal species (Sato 1992). In some algae, such as Chlamydomonas reinhardtii, PC is completely replaced by the betaine lipid DGTS. In others, such as C. minutissima, high amounts of DGTS and PC were detected, while in Chlorella vulgaris DGTS could not be detected (Giroud et al. 1988; Sato 1992; Haigh et al. 1996). Different eukaryotic phytoplankton species respond differently to salt stress in terms of DGTS content. Unlike in this study, where we observed a slight increase in the relative proportion of DGTS content with a decrease in salinity, the haptophyte Isochrysis galbana preserves the relative proportion of DGTS over a wide range of salinity (Cañavate et al. 2020). The fact that DGTS has the greatest diversity of molecular species indicates the importance of this lipid class to D. tertiolecta (Fig. 5, Table S4). The localization of DGTS within the cell has not yet been fully elucidated. Experiments with the green alga C. reinhardtii (Janero and Barrnett 1982) have shown that ~ 40% of cellular DGTS is localized in the thylakoid membrane. In contrast, Sheffer et al. (1986) suggested that DGTS is not present in significant amounts in the thylakoid membrane but is a major component of the plasma-membrane (23.5%) of the green alga D. salina. A similar result was reported for D. salina grown at different salinities, where DGTS accounted for 19.4, 16.0 and 26.8% of the total lipids in the plasma membrane at salinities ~ 50, 100, and 200 PSU, respectively (Peeler et al. 1989). The high molecular diversity of DGTS that we identified may indicate its localization in membranes of different cell organelles. However, the proposed replacements of PC by DGTS suggest a major localization in the plasma membrane.
An increase in phospholipid PI with decreasing salinity can be explained by its role in controlling membrane trafficking and lipid signaling (Peeler et al. 1989; De Craene et al. 2017). We assume that in a hyposaline environment, synthesis of PI is stimulated to allow D. tertiolecta to acclimate to salinity decrease and that one molecular species (PI 18:1/16:0) appears to be important for this alga. The same PI species was detected in D. tertiolecta grown under different stress conditions (Kim et al. 2013, 2015).
Fatty acid remodeling of polar lipid species in response to salinity decrease
The fatty acid composition of the polar lipids of D. tertiolecta is consistent with the reports of Evans and Kates (1984). The redistribution of fatty acyl moieties within a single lipid class is reflected in the changes in the degree of unsaturation as salinity decreases from 38 to 3 PSU, and for MGDG, PC and DGTS these changes are already present at 20 PSU (Fig. 6). The observed high MGDG unsaturation appears to be essential for proper functioning of the thylakoid membranes in D. tertiolecta, and this becomes even more important with decreasing salinity. The most abundant molecular species MGDG 18:3/16:4 increases with decreasing salinity at the expense of MGDG 18:2/16:3 (Fig. 3a, Table S4). We can conclude that MGDG desaturase acts on MGDG 18:2/16:3 in response to the downward shift in salinity. Unlike in the case of hyposalinity, no MGDG remodeling was detected in D. tertiolecta growing under low-nitrate conditions (Kim et al. 2013).
A decrease in salinity leads to a statistically significant remodeling of SQDG fatty acids (Fig. 3b), whereas the overall unsaturation (Fig. 6) and the fatty acid chain lengths (Table 1) do not change. One function of the glycolipid SQDG is to stabilize Photosystem II protein complexes in the chloroplast membranes of the green algae Chlamydomonas (Minoda et al. 2003). Noh et al. (2020) reported that the marine Synechocystis strain contains relatively longer fatty acyl chains in SQDG than the freshwater Synechocystis strain. Growth of D. tertiolecta under high light and nitrogen starvation did not cause significant changes in SQDG (Kim et al. 2013), nor under nitrate-replete and nitrate-depleted conditions (Kim et al. 2015).Thus, we assume that the stable fatty acid composition (total unsaturation and chain length) of SQDG is important for the acclimation and survival of D. tertiolecta.
The response of D. tertiolecta to a decrease in salinity also includes a remodeling of DGDG fatty acids (Fig. 3c, Table S4) that includes decreased unsaturation (Fig. 6), an increasing proportion of SFA with a decreasing PUFA proportion, and an increase in fatty acid chain length from C16 to C18 (Table 1). An extensive search of the published literature did not reveal an explanation for the decrease in DGDG unsaturation in response to a decrease in salinity.
The major PG species of D. tertiolecta is PG 16:1/18:3 (Fig. 4a, Table S4). Salinity decrease caused a decrease in the unsaturation of PG fatty acids (Fig. 6), the replacement of PG 16:1/18:3 and PG 16:0/18:3 by PG 16:0/18:2 (Fig. 4a, Table S4), the replacement of MUFA by SFA, whereas the PUFA content did not change (Table 1). This restructuring did not change the ratio of C16 and C18 fatty acids. Furthermore, PG remodeling was not detected in nitrate-deficiency condition (Kim et al. 2013, 2015). The reduction in the unsaturation of PG and DGDG fatty acids observed here in response to a decrease in salinity suggests a lower nutritional value of D. tertiolecta for higher trophic levels.
Unlike other detected polar lipids, PC is not characterized by the dominance of a single molecular species. The response of PC to a decrease in salinity, including fatty acid remodeling (Fig. 4b, Table S4), decrease in unsaturation (Fig. 6), and increased proportion of MUFA on expense of PUFA (Table 1) are small, but statistically significant. The relative invariability of PC total unsaturation and fatty acid chain lengths was found over a wide range of salinity in two estuaries studied and was explained by the maintaining the estuarine plankton cell integrity and preserving the physicochemical properties of the membranes through low variability in PC unsaturation and fatty acid chain length (Vrana Špoljarić et al. 2021, 2020). We assume that the same conclusion applies to the growth of D. tertiolecta at different salinities.
Acclimations to lower salinity include complex remodeling of DGTS (Fig. 6, Table 1), including reduced unsaturation and fatty acids shortening. These indicate decreased membrane fluidity involving DGTS. Similarly, intense DGTS remodeling occurred during growth of D. tertiolecta in nitrate-deficient conditions (Kim et al. 2015).
The observed decrease in PUFA (α-linolenic acid) in PG, DGTS, SQDG, and PC with decreasing salinity, suggests that the decrease in salinity reduces/inactives membrane-bound enzymes that synthesize PUFA via the aerobic or anaerobic pathway that are involved in α-linolenic acid biosynthesis (Certik and Shimizu 1999). In microorganisms, most of the PUFA is stored in the form of triacylglycerol. The fatty acyl chains are incorporated into the phospholipid PA before they are available for the biosynthesis of diacylglycerol and triacylglycerol (Frentzen 1993). The diacylglycerols are further used for the synthesis of glycolipids (Kalisch et al. 2016). We can conclude that acclimation to salinity decrease in D. tertiolecta diverts synthesis of α-linolenic acid to incorporation into the glycolipids DGDG and MGDG to maintain membrane integrity at the expense of decreasing α-linolenic acid in other lipid classes. However, salinity decrease in D. tertiolecta has a negative effect on total α-linolenic acid in the polar lipids. Since α-linolenic acid is the most abundant omega-3 fatty acid, if not the only PUFA, in the polar cell lipids, the reduction of this fatty acid will have negative impact on the higher trophic levels as D. tertiolecta will be less nutritious food for them.
One of the possible strategies of microalgae to cope with stress is to alter the fatty acid chain length (Goss and Wihelm 2009; Kim et al. 2013). It seems that this acclimation mechanism does not play a major role in the D. tertiolecta hypoosmotic acclimation, as we only observed an increase in C18 fatty acids in DGDG and their decrease in DGTS.
In addition, lipid remodeling could also affect cell surface properties and thus functional cell behavior at interfaces (Novosel et al. 2022b). The cells of D. tertiolecta change their mechanical properties from stiffer and hydrophobic in the exponential phase to less stiff and hydrophilic in the stationary phase of growth (Pillet et al. 2019). Also, the stiffness of D. tertiolecta cells decreases and hydrophilicity increases as the growth temperature increases (Novosel et al. 2021, 2022a). The observed mechanical and chemical changes in D. tertiolecta cells may indicate molecular modification of the cell envelope as well as changes in the lipid composition of cell membranes. Thus, the interplay between lipid composition and cell mechanics would allow us to better understand the acclimation/adaptation mechanism of microalgae cells to hyposaline conditions.
Conclusion
Dunaliella tertiolecta acclimates to growth under hypoosmotic conditions (3 PSU) with a significant retardation of growth and reproduction. Acclimation to salinity of 3 PSU involves statistically significant cellular lipid accumulation, remodeling of polar lipid classes and fatty acid composition. The major lipid responses, seen even at 20 PSU, include increase in MGDG and decrease in PG in the total polar lipids. These suggest that these two polar lipids play the most important role in D. tertiolecta acclimation to hyposaline environment. So, we hypothesize that this microalga is genetically adapted through membrane lipid composition to ensure survival under large salinity fluctuations. The observed decrease in unsaturated fatty acids in most polar lipids of D. tertiolecta with decreasing salinity and the replacement of PC by DGTS likely plays a role in reducing plasma membrane fluidity and altering permeability to prevent unfavorable inflow and outflow of water and ions into and out of cells. The high molecular diversity of DGTS, the relatively uniform representation of PC species, PI 18:1/16:0 and the dominance of one species among MGDG, SQDG, DGDG, PG, and DGTS characterize the polar lipid composition in a broad salinity range between 38 and 3 PSU. The polar lipid fraction of D. tertiolecta is characterized exclusively by C16 and C18 fatty acids. The change in the fatty acid chain length is not a mechanism of D. tertiolecta to cope with the decrease in salinity. Hyposalinity also causes replacement of the omega-3 (linolenic) fatty acid by saturated palmitic acid in PG and DGTS and causes the absence of MGDG 18:3/18:3 lipid species.
Studies on lipid remodeling in microalgae cells in response to hypoosmotic stress are rarely performed. Therefore, the results of our study are important as they provide a new aspect to the fundamental understanding of how D. tertiolecta cells achieve equilibrium in hyposaline conditions with respect to optimal salinity. As the global climate change and extreme precipitation becomes more frequent, it is important to understand the mechanisms that act in the acclimation and adaptation of microalgae to a sudden drop in salinity. Further lipidomics research should be extended to other microalgae species. From a biotechnological point of view, the result of increased content of non-polar lipids in D. tertiolecta cells under hyposalinity suggests that D. tertiolecta may be a good candidate for biodiesel production. Therefore, lipidomic studies should be performed to investigate the remodeling of non-polar lipids of D. tertiolecta under hyposaline conditions.
Data availability
All datasets generated during this study are available from the corresponding author on reasonable request.
References
Arts MT, Ackman RG, Holub BJ (2001) ‘Essential fatty acids’ in aquatic ecosystems: A crucial link between diet and human health and evolution. Can J Fish Aquat Sci 58:122–137
Bligh E, Dyer W (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka LJ, Brown AD (1974) The salt relations of marine and halophilic species of the unicellular green alga, Dunaliella. The role of glycerol as a compatible solute. Arch Mikrobiol 96:37–52
Borowitzka MA (2018a) Biology of microalgae. In: Levine IA, Fleurence J (eds) Microalgae in health and disease prevention. Academic Press, NY, pp 23–72
Borowitzka MA (2018b) The ‘stress’ concept in microalgal biology-homeostasis, acclimation and adaptation. J Appl Phycol 30:2815–2825
Borowitzka MA (2013) Dunaliella: biology, production, and markets. In: Richmond A, Hu Q (eds) Handbook of microalgal culture. John Wiley & Sons, London, pp 359–368
Borowitzka MA, Siva CJ (2007) The taxonomy of the genus Dunaliella (Chlorophyta, Dunaliellales) with emphasis on the marine and halophilic species. J Appl Phycol 19:567–590
Cañavate JP, Hachero-Cruzado I, Pérez-Gavilán C, Fernández-Díaz C (2020) Lipid dynamics and nutritional value of the estuarine strain Isochrysis galbana VLP grown from hypo to hyper salinity. J Appl Phycol 32:3749–3766
Certik M, Shimizu S (1999) Biosynthesis and regulation of microbial polyunsaturated fatty acid production. J Biosci Bioeng 87:1–14
Cheng L, Trenberth KE, Gruber N, Abraham JP, Fasullo JT, Li G, Mann ME, Zhao X, Zhu J (2020) Improved estimates of changes in upper ocean salinity and the hydrological cycle. J Clim 33:10357–10381
De Craene J-O, Bertazzi DL, Bär S, Friant S (2017) Molecular sciences phosphoinositides, major actors in membrane trafficking and lipid signaling pathways. J Mol Sci 18:634
Du ZY, Benning C (2016) Triacylglycerol accumulation in photosynthetic cells in plants and algae. Subcell Biochem 86:179–205
Eichenberger W (1993) Betaine lipids in lower plant. Distribution of DGTS, DGTA and phospholipids, and the intracellular localization and site of biosynthesis of DGTS. Plant Physiol Biochem 31:213–221
Evans RW, Kates M (1984) Lipid composition of halophilic species of Dunaliella from the dead sea. Arch Microbiol 140:50–56
Falkowski PG, Raven JA (2007) Aquatic photosynthesis, 2nd edn. Princeton Univ Press, Princeton, p 484
Fields MW, Hise A, Lohman EJ, Bell T, Gardner RD, Corredor L, Moll K, Peyton BM, Characklis GW, Gerlach R (2014) Sources and resources: Importance of nutrients, resource allocation, and ecology in microalgal cultivation for lipid accumulation. Appl Microbiol Biotechnol 98:4805–4816
Frentzen M (1993) Acyltransferases and triacylgycerols. In: Moore TS (ed) Lipid metabolism in plants. CRC Press, Boca Raton, pp 195–230
Furt F, Simon-Plas F, Mongrand S (2011) Lipids of the plant plasma membrane. Plant Cell Monogr 19:3–30
Garab G (2014) Hierarchical organization and structural flexibility of thylakoid membranes. Biochim Biophys Acta - Bioenerg 1837:481–494
Gašparović B, Kazazić SP, Cvitešić A, Penezić A, Frka S (2015) Improved separation and analysis of glycolipids by Iatroscan thin-layer chromatography-flame ionization detection. J Chromatogr A 1409:259–267
Gašparović B, Kazazić SP, Cvitešić A, Penezić A, Frka S (2017) Corrigendum to “Improved separation and analysis of glycolipids by Iatroscan thin-layer chromatography–flame ionization detection” [J. Chromatogr. A 1409 (2015) 259–267]. J Chromatogr A 1521:168–169
Ghoshal D, Mach D, Agarwal M, Goyal A, Goyal A (2002) Osmoregulatory isoform of dihydroxyacetone phosphate reductase from Dunaliella tertiolecta: Purification and characterization. Protein Expr Purif 24:404–411
Gilmour DJ, Hipkins MF, Boney AD (1984) The effect of decreasing the external salinity on the primary processes of photosynthesis in Dunaliella tertiolecta. J Exp Bot 35:28–35
Ginzburg M (1988) Dunaliella: a green alga adapted to salt. Adv Bot Res 14:93–183
Giroud C, Gerber A, Eichenberger W (1988) Lipids of Chlamydomonas reinhardtii. Analysis of molecular species and intracellular site(s) of biosynthesis. Plant Cell Physiol 29:587–595
Goss R, Wihelm C (2009) Lipids in algae, lichens and mosses. In: Wada H, Murata N (eds) Lipids in photosynthesis: essential and regulatory functions. Springer, Dordrecht, pp 117–137
Guillard RR, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Guillard RRL (1975) Culture of phytoplankton for feeding marine invertebrates. In: Culture of Marine Invertebrate Animals. Plenum Press, NY pp 29–60
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hadi MR, Shariati M, Afsharzadeh S (2008) Microalgal biotechnology: Carotenoid and glycerol production by the green algae Dunaliella isolated from the Gave-Khooni salt marsh, Iran. Biotechnol Bioprocess Eng 13:540–544
Haigh GW, Yoder TF, Ericson L et al (1996) The characterisation and cyclic production of a highly unsaturated homoserine lipid in Chlorella minutissima. Biochim Biophys Acta - Lipids Lipid Metab 1299:183–190
Hamburger C (1905) Zur Kenntnis der Dunaliella salina und einer Amöbe aus Salinenwasser von Cagliari. Arch Protistenk 6:111-130, Plate 111
Harwood JL, Guschina IA (2009) The versatility of algae and their lipid metabolism. Biochimie 91:679–684
Hellebust JA (1985) A comparative study of sodium and osmotic requirements for growth and nutrient uptake of two related green flagellates, Dunaliella tertiolecta and Chlamydomonas pulsatilla. Arch Microbiol 143:11–14
Jahnke LS, White AL (2003) Long-term hyposaline and hypersaline stresses produce distinct antioxidant responses in the marine alga Dunaliella tertiolecta. J Plant Physiol 160:1193–1202
Janero DR, Barrnett R (1982) Isolation and characterization of an ether-linked homoserine lipid from the thylakoid membrane of Chlamydomonas reinhardtii 137+. J Lipid Res 23:307–316
Kalisch B, Dörmann P, Hölzl G (2016) DGDG and glycolipids in plants and algae. In: Nakamura Y, Li-Beisson Y (eds) Lipids in plant and algae development. Subcellular Biochemistry. Springer, Cham, pp 51–83
Kim SH, Ahn HM, Lim SR, Hong SJ, Cho BK, Lee H, Lee CG, Choi HK (2015) Comparative lipidomic profiling of two Dunaliella tertiolecta strains with different growth temperatures under nitrate-deficient conditions. J Agric Food Chem 63:880–887
Kim SH, Liu KH, Lee SY, Hong SJ, Cho BK, Lee H, Lee CG, Choi HK (2013) Effects of light intensity and nitrogen starvation on glycerolipid, glycerophospholipid, and carotenoid composition in Dunaliella tertiolecta culture. PLoS ONE 8:e72415
Kirst GO (1990) Salinity tolerance of eukaryotic marine algae. Annu Rev Plant Physiol 41:21–53
Kobayashi K (2016) Role of membrane glycerolipids in photosynthesis, thylakoid biogenesis and chloroplast development. J Plant Res 2018 1294 129:565–580
Liang MH, Qv XY, Chen H et al (2017) Effects of salt concentrations and nitrogen and phosphorus starvations on neutral lipid contents in the green microalga Dunaliella tertiolecta. J Agric Food Chem 65:3190–3197
Liu W, Ming Y, Li P, Huang Z (2012) Inhibitory effects of hypo-osmotic stress on extracellular carbonic anhydrase and photosynthetic efficiency of green alga Dunaliella salina possibly through reactive oxygen species formation. Plant Physiol Biochem 54:43–48
Lu N, Wei D, Chen F, Yang S (2012a) Lipidomic profiling and discovery of lipid biomarkers in snow alga Chlamydomonas nivalis under salt stress. Eur J Lipid Sci Technol 114:253–265
Lu N, Wei D, Jiang X, Chen F, Yang ST (2012b) Regulation of lipid metabolism in the snow alga Chlamydomonas nivalis in response to NaCl stress: An integrated analysis by cytomic and lipidomic approaches. Process Biochem 47:1163–1170
Mansour MMF (2012) (2012) Plasma membrane permeability as an indicator of salt tolerance in plants. Biol Plant 571:1–10
Minoda A, Sonoike K, Okada K et al (2003) Decrease in the efficiency of the electron donation to tyrosine Z of photosystem II in an SQDG-deficient mutant of Chlamydomonas. FEBS Lett 553:109–112
Mishra A, Mandoli A, Jha B (2008) Physiological characterization and stress-induced metabolic responses of Dunaliella salina isolated from salt pan. J Ind Microbiol Biotechnol 35:1093–1101
Moore T (1982) Phospholipid biosynthesis. Annu Rev Plant Physiol 33:235–259
Murakami H, Nobusawa T, Hori K, Shimojima M, Ohta H (2018) Betaine lipid is crucial for adapting to low temperature and phosphate deficiency in Nannochloropsis. Plant Physiol 177:181–193
Neelam S, Subramanyam R (2013) Alteration of photochemistry and protein degradation of photosystem II from Chlamydomonas reinhardtii under high salt grown cells. J Photochem Photobiol B 124:63–70
Noh YJ, Lee H, Hong SJ, Lee H, Cho BK, Lee CG, Choi HK (2020) Comparative primary metabolic and lipidomic profiling of freshwater and marine Synechocystis strains using by GC-MS and nanoESI-MS analyses. Biotechnol Bioprocess Eng 25:308–319
Novak T, Godrijan J, Pfannkuchen DM, Djakovac T, Medić N, Ivančić I, Mlakar M, Gašparović B (2019) Global warming and oligotrophication lead to increased lipid production in marine phytoplankton. Sci Total Environ 668:171–183
Novosel N, Ivošević DeNardis N (2021) Structural features of the algal cell determine adhesion behavior at a charged interface. Electroanalysis 33:1436–1443
Novosel N, Mišić Radić T, Zemla J, Lekka M, Čačković A, Kasum D, Legović T, Žutinić P, Gligora Udovič M, Ivošević Denardis N (2022a) Temperature-induced response in algal cell surface properties and behaviour: an experimental approach. J Appl Phycol 34:243–259
Novosel N, Mišić Radić T, Levak Zorinc M, Zemla J, Lekka M, Vrana I, Gašparović B, Horvat L, Kasum D, Legović T, Žutinić P, Gligora Udovič M, Ivošević DeNardis N (2022b) Salinity induced chemical, mechanical and behavioral changes in marine microalgae. J Appl Phycol. https://doi.org/10.1007/s10811-022-02734-x
Oliveira L, Bisalputra T, Anita NJ (1980) Ultrastructural observation of the surface coat of Dunaliella tertiolecta from staining with cationic dyes and enzyme treatments. New Phytol 85:385–392
Oren A (2005) A hundred years of Dunaliella research: 1905–2005. Saline Systems 1:1–14
Pal D, Khozin-Goldberg I, Didi-Cohen S, Solovchenko A, Batushansky A, Kaye Y, Sikron N, Samani T, Fait A, Boussiba S (2013) Growth, lipid production and metabolic adjustments in the euryhaline eustigmatophyte Nannochloropsis oceanica CCALA 804 in response to osmotic downshift. Appl Microbiol Biotechnol 97:8291–8306
Peeler TC, Stephenson MB, Einspahr KJ, Thompson GA (1989) Lipid characterisation of an enriched plasma membrane fraction of Dunaliella salina grown in media of varying salinity. Plant Physiol 89:970–976
Pick U (2002) Adaptation of the halotolerant alga Dunaliella to high salinity. In: Läuchli A, Lüttge U (eds) Salinity: Environment - Plants - Molecules. Springer, Dordrecht, pp 97–112
Pillet F, Dague E, Pečar Ilić J, Ružić I, Rols M-P, Ivošević DeNardis N (2019) Changes in nanomechanical properties and adhesion dynamics of algal cells during their growth. Bioelectrochemistry 127:154–162
Richmond A (1986) Cell response to environmental factors. In: Richmond A (ed) Handbook of microalgal mass culture. CRC Press, Boca Raton, pp 89–95
Rizwan M, Mujtaba G, Memon SA, Lee K (2020) Influence of salinity and nitrogen in dark on Dunaliella tertiolecta’s lipid and carbohydrate productivity. Biofuels 13:475–481
Rizwan M, Mujtaba G, Rashid N, Lee K (2017) Enhancing lipid production of Dunaliella tertiolecta by manipulating the interactive effect of salinity and nitrogen. Chem Biochem Eng Q 31:199–207
Sato N (1992) Betaine lipids. Bot Mag Tokyo 105:185–197
Sato N, Furuya M (1985) Distribution of diacylglyceryltrimethylhomoserine and phosphatidylcholine in non-vascular green plants. Plant Sci 38:81–85
Sheffer M, Fried A, Gottlieb HE, Tietz A, Avron M (1986) Lipid composition of the plasma-membrane of the halotolerant alga, Dunaliella salina. Biochim Biophys Acta - Biomembr 857:165–172
Shetty P, Gitau MM, Maróti G (2019) Salinity stress responses and adaptation mechanisms in eukaryotic green microalgae. Cells 8:cells8121657
Svetličić V, Ivošević N, Kovač S, Žutić V (2001) Charge displacement by adhesion and spreading of a cell. Bioelectrochemistry 53:79–86
Tammam AA, Fakhry EM, El-Sheekh M (2011) Effect of salt stress on antioxidant system and the metabolism of the reactive oxygen species in Dunaliella salina and Dunaliella tertiolecta. African J Biotechnol 10:3795–3808
Takagi M, Karseno, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226
Thompson PA, Guo M-x, Harrison PJ, Whyte JNC (1992) Effects of variation in temperature. II. On the fatty acid composition of eight species of marine phytoplankton. J Phycol 28:488–497
Tietz S, Leuenberger M, Höhner R et al (2020) A proteoliposome-based system reveals how lipids control photosynthetic light harvesting. J Biol Chem 295:1857–1866
Vrana Špoljarić I, Novak T, Gašparović B, Kazazić SP, Čanković M, Ljubešić Z, Hrustić E, Mlakar M, Du J, Zhang R, Zhu Z (2021) Impact of environmental conditions on phospholipid fatty acid composition: implications from two contrasting estuaries. Aquat Ecol 55:1–20
Wada H, Murata N (2007) The essential role of phosphatidylglycerol in photosynthesis. Photosynth Res 92:205–215
Wang T, Ge HY, Liu TT, Tian XW, Wang ZJ, Guo MJ, Chu J, Zhuang YP (2016) Salt stress induced lipid accumulation in heterotrophic culture cells of Chlorella protothecoides: Mechanisms based on the multi-level analysis of oxidative response, key enzyme activity and biochemical alteration. J Biotechnol 228:18–27
Xiang Q, Wei X, Yang Z et al (2021) Acclimation to a broad range of nitrate strength on a euryhaline marine microalga Tetraselmis subcordiformis for photosynthetic nitrate removal and high-quality biomass production. Sci Total Environ 781:146687
Yao S, Lu J, Sárossy Z, Baggesen C, Brandt A, An Y (2016) Neutral lipid production in Dunaliella salina during osmotic stress and adaptation. J Appl Phycol 28:2167–2175
Yu B, Benning C (2003) Anionic lipids are required for chloroplast structure and function in Arabidopsis. Plant J 36:762–770
Funding
This work is supported by the Croatian Science Foundation Projects IP-2018–01-5840 and IP-11–2013-8607 and Slovenian Research Agency programme P1-0143 and project J4-1773.
Author information
Authors and Affiliations
Contributions
I. Vrana: lipidomics analysis, data analysis, creating figures, writing first draft and editing the manuscript; S. Bakija Alempijević: culture experiments, microscopy, writing and editing the manuscript; N. Novosel: culture experiments, writing and editing the manuscript; N. Ivošević DeNardis: experimental set-up, funding, writing and editing the manuscript; D. Žigon: lipidomics analysis, writing and editing the manuscript; N. Ogrinc: funding, writing and editing the manuscript; B. Gašparović: original concept, funding, writing and editing the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vrana, I., Bakija Alempijević, S., Novosel, N. et al. Hyposalinity induces significant polar lipid remodeling in the marine microalga Dunaliella tertiolecta (Chlorophyceae). J Appl Phycol 34, 1457–1470 (2022). https://doi.org/10.1007/s10811-022-02745-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-022-02745-8