Abstract
Picocystis salinarum is a globally widespread picoplanktonic green alga of saline lakes. This tiny alga has been recorded in four continents, and in some cases, it has become the dominant phytoplankton species. We examined the ecophysiology of a Kenyan strain of P. salinarum, collected from Lake Nakuru. The photosynthetic activity of the species was measured in seventy-two combinations of light intensity and temperature. The photosynthetic activity was low along the temperature gradient with an optimum at 31.9 °C; it varied between 0.097 and 1.233 μg C μg−1 Chl a h−1. The ability of P. salinarum to utilize low light intensity (α varied between 0.0061 and 0.1 (μg C μg−1 Chl a h−1) (μmol photons m−2 s−1)−1) and its susceptibility to photoinhibition at different temperatures confirm the species’ preference for low light intensity, which is already shown by its Ik values (1.0–89.3 μmol photons m−2 s−1). The salinity tolerance of P. salinarum, more specifically the effect of changes in the concentration of chloride (NaCl) and carbonate forms (Na2CO3 and NaHCO3), was investigated in continuous cultures. The dominant ion of the medium greatly affected the growth of P. salinarum: significantly higher growth rates were recorded in carbonate form-dominated media as compared to chloride-dominated ones; the highest growth rate was observed at the highest concentration. The observed physiological properties (slow growth, low photosynthetic activity) are not typical of a dominant species; however, the high conductivity tolerance could be a key factor explaining the success of P. salinarum.
Similar content being viewed by others
Introduction
The soda lakes and pans of the East African Rift Valley serve as very special habitats because of their extreme conditions such as high water temperature (18.9–32.0 °C), high and rapidly changing conductivity (23.1–96.2 mS cm−1), and high alkalinity (pH is usually about 10) (Vareschi 1982; Jirsa et al. 2013; Schagerl et al. 2015; Schagerl 2016). The Lesser Flamingo (Phoeniconaias minor Geoffroy Saint-Hilaire 1798) is the flagship species of these habitats (Childress et al. 2008). The main food resource of these birds is the spirally twisted cyanobacterium, Limnospira fusiformis (Voronichin) Nowicka-Krawczyk, Mühlsteinová & Hauer (Nowicka-Krawczyk et al. 2019), syn. Arthrospira fusiformis (Voronichin) Komárek & J.W.G. Lund. These specialized feeders are equipped with bill lamellae that enable them to filter food in the size range of 15–800 μm from the water. In many publications, this cyanobacterium is named “Spirulina” in diverse invalid designations, mostly as “Spirulina platensis.” This cyanobacterium regularly attains bloom levels in these waterbodies (Jenkin 1957; Vareschi 1978; Kaggwa et al. 2013). A strong relationship between the biomass of Limnospira fusiformis and the abundance of Lesser Flamingos has been reported in the soda lakes of East Africa (Vareschi 1978; Tuite 2000; Krienitz and Kotut 2010; Kaggwa et al. 2013). In the absence of L. fusiformis, Lesser Flamingos survive by grazing on diatoms or other microphytes of acceptable size range (Krienitz et al. 2016a). Alternatively, they migrate to other soda lakes and pans of East Africa in search of a suitable food resource (Krienitz et al. 2016b).
In Lake Nakuru and Lake Bogoria, the population of L. fusiformis periodically crashes and is replaced by the tiny green alga Picocystis salinarum R.A. Lewin, member of the class Picocystophyceae (Lopes dos Santos et al. 2017). Picoplankton is a rarely examined group of the phytoplankton of the East African soda lakes as most past studies focused on the food species of the Lesser Flamingos. Picocystis was first observed in Lake Bogoria in 2006 (Krienitz and Kotut 2010) and Lake Nakuru in 2010 (Krienitz et al. 2012). Picocystis salinarum forms subdominant populations in the soda lakes of the East African Rift Valley (Table 1) where it usually contributes little to the total biomass. Its biomass contribution rose to more than 4% during the dry season in 2009 in Lake Nakuru when the lake was about to dry out, before heavy rains refilled it. Following the collapse of the Limnospira population in January 2010, P. salinarum with a biomass contribution of 53–68% of the total biomass became dominant (Krienitz et al. 2012).
Picocystis salinarum was first described from a saline pond at the San Francisco Salt Works, California, USA, as green spherical cells with a diameter of 2–3 μm; without flagella, basal bodies, and superficial body scales; and living in saline waters. The major pigments present in the species are chlorophylls a and b and the carotenoids alloxanthin, diatoxanthin, and monadoxanthin (Lewin et al. 2000; Lopes dos Santos et al. 2016). Identification of the species is difficult; only the trilobite cell morphology (described by Roesler et al. (2002) as being reminiscent of Mickey Mouse) in older cultures or sometimes field samples can be helpful in its visual identification (Lewin et al. 2000; Krienitz et al. 2012).
Picocystis strains have also been recorded in several other saline waterbodies: in North Africa (Rihab et al. 2017); in the North American region; in Mono Lake, USA (Roesler et al. 2002); in the South American region; in Peru (Tarazona Delgado et al. 2017); in an Asian soda lake in Inner Mongolia (Hollibaugh et al. 2001; Fanjing et al. 2009); in Lake Sambhar, India (Krienitz 2018); and in Lake Dziani Dzaha (Mayotte Island, Indian Ocean; Cellamare et al. 2018; Bernard et al. 2019). Phylogenetic analysis of strains from Africa and India revealed that all the strains belong to one and the same species, Picocystis salinarum.
The aim of this study was to establish the key of the success of this morphologically very simple organism in such special environments as soda lakes and to determine what makes it the dominant species of phytoplankton in some cases. We hypothesized that P. salinarum has the following abilities to occupy this kind of habitats:
-
I.
Since the species occurs in a wide temperature range (from under ice to hot springs) and becomes dominant in productive habitats such as the soda lakes of East Africa, it probably has a high photosynthetic rate along a wide temperature range between 10 and 45 °C.
-
II.
As the species can survive and become dominant in light-limited environments (under ice, turbid water), it is expected to be efficient in light utilization in various physical environments.
-
III.
Since the species occurs in alkaline saline or even hypersaline waters of four continents, it tolerates and/or grows fast under different conductivities.
To test these hypotheses, the photosynthetic activity of P. salinarum was examined in a wide range of both temperature and light intensity, and its growth was investigated in fourteen culture media with different conductivities resulting from two different dominant ions.
Materials and methods
Strain
To conduct photosynthesis and growth experiments, we used a Picocystis salinarum (KR 2010/2) strain from the collection of the Leibniz-Institute of Freshwater Ecology and Inland Fisheries, which was collected from Lake Nakuru, Kenya, in 2010. The strain was deposited at the Culture Collection of Algae of Charles University in Prague (CAUP) under the number M301. Taxonomic identity was confirmed by molecular phylogenetic analysis (Krienitz et al. 2012). The sequence of the small-subunit (SSU) rRNA gene is stored at the National Center for Biotechnology Information (NCBI) under the accession number HM990668.
A new medium developed by Shafik et al. (2014) to cultivate algae species from East African alkaline saline lakes was applied (M0 in Table 2) to maintain clean cultures and to carry out measurements.
For photosynthesis and chemostat measurements, P. salinarum strain was grown in a batch culture in the Alga Culturing Laboratory of the Department of Limnology (University of Pannonia, Veszprém). The stock and experimental cultures were maintained at 20 ± 1 °C under photosynthetic active radiation (PAR) of 65 μmol photons m−2 s−1 and a 14:10 light:dark cycle in Erlenmeyer flasks (stock culture in 1 L and experimental cultures in 5 L).
Photosynthesis measurements
The photosynthetic characteristics of P. salinarum were examined in 72 combinations of a wide range of both temperature and light intensity to determine the optimum conditions for the species and its temperature and light intensity tolerance ranges. Measurements were carried out in a special incubation system, the photosynthetron (Üveges et al. 2011). The photosynthetron is an aquarium system filled up with distilled water. The instrument consists of nine cells (Fig. 1) with different light intensities (0; 7.5; 30; 85; 175; 300; 450; 900, and 1200 μmol photons m−2 s−1). PAR was provided by daylight tubes (Tungsram F74), and light intensities were measured with LI 1400 (LI-COR) equipped with a spherical (4π) quantum sensor (US-SQS/L, Heinz Walz GmbH). Specific measuring temperatures were provided by circulating the distilled water in the photosynthetron with a circulating water bath (Neslab RTE-211) in the temperature range of 10–45 °C (with 5 °C increments).
Theoretical diagram of photosynthetron: solid lines represent glass walls, and dotted lines represent mirror walls of the cells of the aquarium system. A circulating water bath (Neslab RTE-211) is responsible for the specific temperature of the instrument via circulating distilled water in the photosynthetron. PAR is provided by daylight tubes (Tungsram F74); a different light intensity is set with the number of light tubes and shielding foil
We followed a protocol previously employed by others who used this instrument to carry out photosynthesis measurements (Üveges et al. 2012; Lázár et al. 2015; Lengyel et al. 2015, 2020). Prior to carrying out the photosynthetic measurements, cultures and fresh medium (M0) were placed in a plastic chamber with an approximate volume of 15 L. Photosynthesis measurements started when the mass culture reached exponential growth phase. Growth was followed by OD measurements at 750 nm with a Metertech SP-8001 UV-VIS spectrophotometer from a subsample of the homogenized culture.
After homogenization, the culture was divided into Karlsruhe flasks, with an approximate volume of 250 mL (we used this kind of flask for the measurements to avoid gas exchange with the environment) in three replicates at every light intensity (in each measuring cell of the photosynthetron). Photosynthetic measurements were started at 10 °C with a 1-h pre-incubation in the dark. Photosynthetic activity of the species was followed by measuring dissolved oxygen (DO) concentration with an IntelliCAL LDO101 sensor (Hach Lange). DO was measured at the beginning of the experiment (t = 0 h), then after 1 h (t = 1 h) and if necessary after 2 h (t = 2 h) (depending on the density of the culture). After the measurement at 10 °C, the culture was homogenized in the plastic vessel and divided into the flasks again. The temperature of the photosynthetron was raised up to 15 °C, and after the repeated 1-h pre-incubation, the photosynthetic activity was measured again. This process was repeated at different measuring temperatures (20–25–30–35–40–45 °C). Following the photosynthesis measurement, chlorophyll-a concentration was measured in ethanol extracts according to MSZ ISO 10260:1993 from a subsample of the homogenized culture at every temperature.
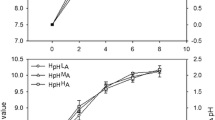
Respiration and gross and net photosynthesis were determined according to Wetzel and Likens (2000). Two equations were used to determine the photosynthetic parameters (see description and units in Table 3) of the species: in the absence of photoinhibition, photosynthetic parameters were calculated according to Webb et al. (1974). When photoinhibition was observed, β (photoinhibition parameter) and the other parameters were calculated according to Platt et al. (1980). Compensation light intensities were calculated according to Pálmai et al. (2018). To calculate the optimum temperature for the different photosynthetic parameters, Gaussian and exponential curves were fitted. Exponential curve was used to describe the changes in the biomass-specific respiration (RB) of P. salinarum, and we used the absolute values of RB (Fig. 2). All curves were fitted using GraFit software (Leatherbarrow 2009).
Temperature dependence of four photosynthetic parameters of P. salinarum from Lake Nakuru in laboratory experiment. PBmax, biomass-specific maximal photosynthetic production (μg C μg−1 Chl a h−1); Ps, maximal photosynthetic production obtained in the absence of photoinhibition; without photoinhibition, it is equal to PBmax (μg C μg−1 Chl a h−1); Ik, photoadaptation parameter (μmol photons m−2 s−1); |RB|, absolute value of biomass-specific respiration (μg C μg−1 Chl a h−1)
Chemostat measurements
To determine the effects of conductivity on the growth rate of P. salinarum, experiments were carried out in a continuous algal culturing system (chemostat) described by Shafik et al. (2001). Culturing vessels were placed into an aquarium filled up with distilled water. Specific temperature was provided by a Thermo Scientific AC150-A25 circulating bath. Subsamples from the main culture at an exponential phase were transferred into the culturing vessels. The cultures were first grown in the initial medium (M0) before carrying out tests on the performance of the species under different salt concentrations. Fourteen different culture media were used (Table 2) to test the effect of the different concentrations of chloride (NaCl) and carbonate forms (both Na2CO3 and NaHCO3) on the growth of P. salinarum. Shifts between media of different concentrations were done when the cultures reached steady state; therefore, sample numbers from different media might slightly differ. Effects of the increasing concentration of chloride and carbonate forms were examined separately in two different culture vessels with an approximate volume of 1000 cm3. Experiments were carried out at a temperature of 29 ± 1 °C and a light intensity of 200 μmol photons m−2 s−1 emitted light with a 12:12 dark:light cycle. Light intensity was measured before the experiments in distilled water. During the experiment inside the culturing vessel, light intensity probably decreased according to the self-shading of P. salinarum cells. The growth medium was continuously added with a Masterflex L/S variable-speed drive, at a flow rate of 160 ± 15 mL day−1. The flow rate was determined based on pilot studies, which were carried out on both P. salinarum and L. fusiformis. Cultures were continuously mixed through aeration with filtered air (0.2-μm pore size Millipore membrane was used). The chosen experimental conditions were found to be optimal for the photosynthesis of P. salinarum in the present study and are similar to conditions in the natural habitat of this species.
Changes in biomass were monitored by measuring the optical density of the samples at 750 nm with a spectrophotometer (Metertech SP-8001) as a strong correlation was established between the chlorophyll a concentration and the absorbance at 750 nm (Fig. 3). Samples were taken three times a week. Growth rate (μ) was calculated using the formula of Shafik et al. (1997) as previously described by Novick and Szilard (1950) and Monod (1978):
where A1 is the absorbance of the culture at time t1, A0 is the absorbance of the culture at time t0, and D is the dilution rate.
Statistical analysis
Since assumptions of ANOVA were not met, Kruskal-Wallis tests were carried out to determine if the differences in photosynthetic activity (carbon uptake) values of the different temperature and light intensity treatments were significant. Owing to the unequal number of observations per group, a Dunn test (Dunn 1964) was used for multiple comparisons where p values were adjusted with the Benjamini-Hochberg method.
Because of the unequal variances and sample sizes, Welch’s t test was applied to determine whether the differences in average growth rates under carbonate-dominated and chloride-dominated media were significant. The Spearman rank correlation coefficient was used to assess the relationship between growth rate and conductivity in carbonate- and chloride-dominated media. In the case of both main media types, a one-way analysis of variance (ANOVA) was performed to test whether the modification of carbonate and chloride content (indicated by the different medium subtypes, Table 2) affects significantly the growth rates of P. salinarum. Subsequently, Tukey’s post hoc multiple comparison tests were conducted between each pair of carbonate- and chloride-dominated media. Statistical analyses were carried out using R statistical computing environment (R Core Team 2018).
Results
Photosynthesis-light characteristics
The photosynthesis-light intensity (P-I) curves of P. salinarum (Fig. 4) revealed an increase in photosynthetic activity with increasing temperature. The highest photosynthetic value was measured at 40 °C. A remarkable drop was recorded at the highest experimental temperature of 45 °C. At this temperature, the photosynthetic activity decreased to the value recorded at 30–35 °C. As a confirmation of these trends, a Kruskal-Wallis test revealed a significant effect of temperature treatments (χ2 = 132.08, df = 7, p < 0.001). A Dunn multiple-comparisons test indicated that the photosynthetic activity differed significantly among the majority of the temperature treatments (Table S1 in Supplemental Data).
The biomass-specific maximal production (PBmax) increased between 0.097 and 1.233 μg C μg−1 Chl a h−1 in the temperature range of 10–40 °C. At 45 °C, a remarkable drop in the PBmax to 50% of the maximal photosynthetic activity was recorded. Very small differences between PBmax and Ps (maximal production obtained in the absence of photoinhibition) values were recorded along the temperature gradient (Table 3); the biggest difference was 7.3% (at 40 °C). For these two parameters, the optimum temperature for the species was 31.9 °C (Fig. 2).
As in the case of PBmax, values of photoadaptation parameter (Ik) increased with the increase in temperature too, with a maximum at 40 °C. The optimum light intensity for the species at different temperatures was found to be quite low: it varied between 1 and 89 μmol photons m−2 s−1. The compensation light intensities (Ic) were determined to be around 2 μmol photons m−2 s−1 in the temperature range of 10–30 °C. At higher temperatures (35–45 °C), a remarkable increase was recorded with the highest value of 14 μmol photons m−2 s−1 being computed at 45 °C.
Outstanding light utilization (α) of the species was determined along the temperature gradient. Balanced values were recorded, except at the lowest measured temperature of 10 °C when an extremely high α value was calculated. Increasing level of photoinhibition (β) was recorded along the temperature gradient, with the highest value at 40 °C. The biomass-specific respiration (RB) of P. salinarum increased exponentially with an increase in temperature (Fig. 2).
Photosynthesis-temperature characteristics
The photosynthetic activity of P. salinarum showed a normal distribution along the temperature gradient at each examined light intensity (Fig. 5). Higher photosynthetic activity was observed between 135 and 1800 μmol photons m−2 s−1, in the higher range of the light intensity gradient. The highest biomass-specific photosynthetic activity values were observed at 700 and 350 μmol photons m−2 s−1. The temperature optima of the photosynthetic activity within this range varied between 36.2 and 37.6 °C. At 45 μmol photons m−2 s−1, a decrease in the photosynthetic activity of P. salinarum was noted. The decrease also occurred in the optimum temperature at this light level. At 45 μmol photons m−2 s−1, the optimum temperature was 33.9 °C. Although the Kruskal-Wallis test (χ2 = 29.852, df = 7, p < 0.001) confirmed that light intensity treatments had a significant effect on photosynthetic activity, the Dunn test (Table S2 in Supplemental Data) revealed that only the photosynthetic activity at the lowest light intensity (15 μmol photons m−2 s−1) was significantly lower than at higher light levels along the temperature gradient. The optimum temperature was also lower at this light treatment (29 °C).
Effects of different culture media
Fourteen different media in increasing concentration of carbonate or chloride forms were used to examine the effect of conductivity changes on the growth rate of P. salinarum (Table 2). Welch’s t test indicated that the mean growth rate in the carbonate-dominated media was significantly higher (t = 13.96, df = 329.05, p < 0.001) than that of the chloride-dominated media (Fig. 6).
In the carbonate-dominated medium, Spearman’s rank correlation revealed a strong positive correlation between conductivity and the growth rate of P. salinarum (r = 0.64, p < 0.001). A one-way ANOVA revealed the existence of a significant difference in the growth rates of the different media subtypes (df = 5, F = 53.614, p < 0.001). The mean growth rate of P. salinarum in the initial medium (M0) was 0.131 ± 0.035 day−1. In M1 medium, the mean growth rate was significantly higher (0.160 ± 0.019 day−1). From M1 to M2 (mean growth rate, 0.16 ± 0.027 day−1), a slight, non-significant increase was observed (Fig. 6a, Table S3 in Supplemental Data). The difference in mean growth rates in media M3–M5 was not significant (Table S3 in Supplemental Data). Mean growth rates recorded in M3, M4, and M5 were 0.220 ± 0.024 day−1, 0.214 ± 0.043 day−1, and 0.243 ± 0.027 day−1 respectively.
For the chloride-dominated media, a weak negative correlation between conductivity and the growth rate (r = − 0.26, p < 0.001) was recorded. A one-way ANOVA test revealed that the growth rate of P. salinarum differed significantly in culture media containing different chloride concentrations (df = 8, F = 8.473, p < 0.001). An initial increase in the mean growth rate (M6, 0.143 ± 0.013 day−1; M7, 0.141 ± 0.014 day−1; and M8, 0.166 ± 0.017 day−1) was observed (Fig. 6b). However, whereas the difference between M6 and M7 was not significant, the difference between M7 and M8 was significant (Table S4 in Supplemental Data). A further increase in sodium chloride concentration resulted in a decline in growth rate (Fig. 6b). However, the difference in the growth rate of the species in the subsequent growth media was not significant (Table S4 in Supplemental Data). The mean growth rates registered in the remaining media concentrations were 0.150 ± 0.017 day−1, 0.135 ± 0.024 day−1, 0.130 ± 0.017 day−1, 0.126 ± 0.012 day−1, and 0.123 ± 0.006 day−1 in M9, M10, M11, M12, and M13 respectively.
Discussion
Soda lakes are important natural assets with cultural, economic, scientific, conservation, and ecological values (Williams 2002; Harper et al. 2016; Oduor and Kotut 2016). The alkaline saline lakes of East Africa have been associated with high photoautotrophic primary productivity and are recognized as being among the most productive ecosystems of the world (Talling et al. 1973; Melack and Kilham 1974; Oduor and Schagerl 2007). Phytoplankton productivity in lakes plays an important role in biogeochemical cycles and supply food to heterotrophs. This is especially true for soda lakes of the semi-arid regions because phytoplankton primary production is almost the only carbon supply in these aquatic ecosystems. Poor macrophyte growth is due to both limited rainfall and discharge from inflows as well as to poor light climate of the water column and littoral areas caused by high primary production of phytoplankton (Vareschi 1978, 1982; Cloern 1996; Oduor and Schagerl 2007).
Primary production in Lake Nakuru is usually dominated by the spirally twisted cyanobacterium Limnospira fusiformis, which can double its biomass in 11–20 h (Vonshak et al. 1996) and is able to grow and photosynthesize well under high temperature and light intensity (Kebede and Ahlgren 1996; Pálmai et al. 2013). Hence, the species has the capacity to meet the food needs of a dense population of Lesser Flamingos. In the absence of this cyanobacterium, the birds have to move between soda lakes to find sufficient food supplies (Torzillo and Vonshak 1994).
Due to its fast growth rate and very high level of photosynthetic activity, L. fusiformis is able to maintain a dominant status in Lake Nakuru and other soda lakes of the African Rift Valley. However, as a result of changes in environmental conditions, the population of the cyanobacterium crashes from time to time (Vareschi 1982; Tuite 2000; Krienitz and Schagerl 2016). Melack (1988) offered eight possible reasons for the disruption of Limnospira that include changes in salinity, nutrient concentrations, and water level; competitive displacement by coccoid or flagellated plankton and attacks by cyanophages; or autolysis. A study by Peduzzi et al. (2014) details the role of infections by viruses in the breakdown of Limnospira populations. In one of the crash episodes, the pico-sized chlorophyte, Picocystis salinarum, which is too small for being grazed by Lesser Flamingos, became dominant in Lake Nakuru’s phytoplankton community (Krienitz et al. 2012).
P. salinarum occurs in temperate or warm, carbonate-rich, alkaline saline lakes of four continents (Table 1). The only exception was reported by Fanjing et al. (2009), who found a bloom-forming population of P. salinarum under ice cover. However, the cold adaptation or preference of P. salinarum as described by Fanjing et al. (2009) is contrary to our findings on the temperature preference and the other findings on the occurrences of the species (Table 1). Under 10 °C, the photosynthetic activity of P. salinarum was undetectable with the LDO method even when we used a very high biomass and longer measuring period (~ 1500 μg Chl a L−1, 3–4 h). Hence, bloom formation of the species under ice cover appears to be an exceptional event, which, however, cannot be ruled out since the strain was positively identified by phylogenetic analysis (Fanjing et al. 2009).
The photosynthetic activity of P. salinarum is quite low, especially when compared to the main competitor L. fusiformis, which has higher biomass-specific photosynthetic activity by at least an order of magnitude along the temperature gradient, and also to other bloom-forming cyanobacteria or algal species (Table 4). A similar level of photosynthetic activity was determined for dominant diatom species of shallow alkaline, soda pans in the Carpathian Basin (Lázár et al. 2015; Lengyel et al. 2015). Oduor and Schagerl (2007) and Harding (1997) found high productivity and high PBmax values for phytoplankton in the East African lakes (Table 4). The productivity of these lakes and also the productivity of the dominant species by far exceeded the chlorophyll a-specific photosynthesis of the KR 2010/2 strain of P. salinarum.
The adaptation of P. salinarum to low light intensity was described by Roesler et al. (2002). This adaptation explains its occurrence in the turbid waters of East Africa (Krienitz et al. 2012) and is consistent with its bloom formation under ice (Fanjing et al. 2009). We confirmed the preference of low light levels along wide range of temperature values (10–45 °C). Adaptation to low light level with good light utilization along the temperature gradient allows the species to be successful in light-limited habitats. L. fusiformis, the most important competitor of P. salinarum in the East African soda lakes in terms of its mass production, provides shaded, low light habitat for P. salinarum, thus allowing the picoeukaryote species to survive and in this case to become dominant.
Significant differences in the effect of carbonates and chloride on the growth rate of P. salinarum were confirmed in the present study. L. fusiformis has also been reported to have a higher growth rate in carbonate-dominated media as compared to chloride-dominated ones. However, the mean growth rate of the species showed a negative correlation with salinity increase (Kebede 1997). Tolerance, or even a preference for a high conductivity by P. salinarum, seems to be one of the most important features of the species: Fanjing et al. (2009) recorded the highest growth at a sodium chloride range from 29.2 to 58.4 g L−1 and no growth at a higher concentration range (between 230 and 300 g L−1). The effect of salinity on the growth of the species has also been investigated for a strain from Mono Lake over a wide range, with a peak in the growth rate at 40 ppt (~ 60 mS cm−1) (Roesler et al. 2002). The highest growth rate recorded in the present study in the M5 medium is close to the salinity level of the Mono Lake strain. However, our finding was ~ 1 day−1 lower than that determined by Roesler et al. (2002). Although we employed a higher light intensity in the present study, our findings did not reach the growth rate reported by Shafik et al. (2014). Comparing the findings of Kebede and Ahlgren (1996) and Kebede (1997) on the maximum specific growth rate (1.78 and 2.14 day−1) of the outcompeted L. fusiformis to that of the Kenyan strain of P. salinarum (0.243 day−1), it is evident that the specific growth rate of L. fusiformis is higher by an order of magnitude than that of P. salinarum. However, the growth rate values were strongly dependent on temperature and salinity. Although the present study confirmed that the concentration of both carbonate forms and chloride significantly affected the growth of P. salinarum, this effect was less pronounced than was recorded in previous studies (Roesler et al. 2002; Fanjing et al. 2009). This difference can be explained by a difference in experimental conditions and culture methods. In our study, we used an African strain of P. salinarum and different media with a different culture method. We applied chemostat instead of batch cultures, which provided continuous transition between different media, thus eliminating drastic shifts in conductivity, which favors the acclimation of the species to the new medium. Although an increase in conductivity has a significant effect on the growth of P. salinarum, the impacts of light intensity and temperature appear to be much more important.
Our results and previous examinations show that P. salinarum is the beneficiary of environmental changes in the East African soda lakes: it can survive in rapidly changing environments and can invade new habitats and displace other species. Moreover, it could be a very important species in the ecosystem of other soda lakes: Roesler et al. (2002) found P. salinarum as a good food source for Artemia monica Verrill, 1869 in a grazing experiment.
In the soda lakes of East Africa, L. fusiformis is an important species not only from an ecological and touristic point of view but also economically because it is rich in antioxidants, essential amino acids, minerals, proteins, polyunsaturated fatty acids, and vitamins (Ciferri 1983; Nuhu 2013; Shao et al. 2019). Picocystis, the green alga that periodically replaces L. fusiformis, also has a high potential for biotechnological use. In Picocystis cultures, lipid droplets have been observed in periods of nitrogen limitation, suggesting a potential source of biofuel (Wang et al. 2014). In general, both Limnospira and Picocystis are promising for mass culture in open ponds in the tropical regions (Krienitz et al. 2016c). Open pond culture systems are the most economic options for algal mass culture in arid areas (Borowitzka and Moheimani 2013). Moreover, Picocystis has been reported to be highly tolerant to heavy metal pollution, especially cadmium (Rihab et al. 2017), and as a good bisphenol A remover (Ben Ouada et al. 2018a). P. salinarum with its shade tolerance can survive alongside the dominant cyanobacterium species, especially L. fusiformis (Vareschi 1982; Krienitz et al. 2016a), as the bloom of L. fusiformis provides optimal (light) conditions for the survival of P. salinarum. Rapid changes in the conditions of the physical environment such as the rapidly changing conductivity caused by sudden dry outs and rapid refilling support the expansion of the picoeukaryote and at the same time result in the collapse of the L. fusiformis population (Vareschi 1982; Jirsa et al. 2013). According to Krienitz (2018), the potential advantage of P. salinarum is the higher tolerance of conductivity changes, which does not favor L. fusiformis. Although the performance of the two species (P. salinarum and L. fusiformis) has not been tested in mixed culture, our finding as well as those of Kebede (1997) and Vonshak et al. (1996) confirm the higher conductivity tolerance of P. salinarum that gives the species a competitive edge over L. fusiformis.
References
Arad A, Morton WHH (1969) Mineral springs and saline lakes of the Western Rift Valley, Uganda. Geochim Cosmochim Acta 33:1169–1181
Ben Ouada S, Ben Ali R, Leboulanger C, Ben Ouda H, Sayadi S (2018a) Effect of bisphenol A on the extremophilic microalgal strain Picocystis sp. (Chlorophyta) and its high BPA removal ability. Ecotoxicol Environ Saf 158:1–8
Ben Ouada S, Ben Ali R, Leboulanger C, Zaghden H, Choura S, Ben Ouda H (2018b) Effect and removal of bisphenol a by two extremophilic microalgal strains (Chlorophyta). J Appl Phycol 30:1765–1776
Bernard C, Escalas A, Villeriot N, Agogué H, Hugoni M, Duval C, Carré C, Got P, Sarazin G, Jézéquel D, Leboulanger C, Grossi V, Ader M, Troussellier M (2019) Very low phytoplankton diversity in a tropical saline-alkaline Lake, with co-dominance of Arthrospira fusiformis (cyanobacteria) and Picocystis salinarum (Chlorophyta). Microb Ecol 78:603–617
Borlongan IA, Maeno Y, Kozono J, Endo H, Shimada S, Nishihara GN, Terada R (2019) Photosynthetic performance of Saccharina angustata (Laminariales, Phaeophyceae) at the southern boundary of subarctic kelp distribution in Japan. Phycologia 58:300–309
Borowitzka MA, Moheimani NR (2013) Open pond culture systems. In: Borowitzka MA, Moheimani NR (eds) Algae for biofuels and energy. Springer, Dordrecht, pp 133–152
Cellamare M, Duval C, Drelin Y, Djediat C, Touibi N, Agogué H, Leboulanger C, Ader M, Bernard C (2018) Characterization of phototrophic microorganisms and description of new cyanobacteria isolated from the saline-alkaline crater-lake Dziani Dzaha (Mayotte, Indian Ocean). FEMS Microbiol Ecol 94
Childress B, Nagy S, Hughes B (2008) International single species action plan for the conservation of the lesser flamingo Phoeniconaias minor. C Tech Ser. 18, AEWA tech. Ser. 34 59
Ciferri O (1983) Spirulina, the edible microorganism. Microbiol Rev 47:551–578
Cloern JE (1996) Phytoplankton bloom dynamics in coastal ecosystems: a review with some general lessons from sustained investigation of San Francisco Bay, California. Rev Geophys 34:127–168
Coles JF, Jones RC (2000) Effect of temperature on photosynthesis-light response and growth of four phytoplankton species isolated from a tidal freshwater river. J Phycol 36:7–16
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241
Fanjing K, Qinxian J, Jia E, Mianping Z (2009) Characterization of a eukaryotic picoplankton alga, strain DGN-Z1, isolated from a soda lake in inner Mongolia, China. Nat Resour Environ Issues 15:185–189
Hamisi MI, Lugomela C, Lyimo TJ, Bergman B, Diez B (2017) Plankton composition, biomass, phylogeny and toxin genes in Lake big Momela, Tanzania. Afr J Aquat Sci 42:109–121
Harding WR (1997) Phytoplankton primary production in a shallow, well-mixed, hypertrophic south African lake. Hydrobiologia 344:87–102
Harper DM, Tebbs E, Bell O, Robinson VJ (2016) Conservation and management of East Africa’s soda lakes. In: Schagerl M (ed) Soda lakes of East Africa. Springer Nature, Berlin, pp 345–364
Hollibaugh JT, Wong PS, Bano N, Pak SK, Prager EM, Orrego C (2001) Stratification of microbial assemblages in Mono Lake, California, and response to a mixing event. Hydrobiologia 466:45–60
Jenkin PM (1957) The filter-feeding and food of flamingoes (Phoenicopteri). Philos Trans R Soc B 240:401–493
Jirsa F, Gruber M, Stojanovic A, Omondi SO, Mader D, Körner W, Schagerl M (2013) Major and trace element geochemistry of Lake Bogoria and Lake Nakuru, Kenya, during extreme draught. Geochemistry 73:275–282
Kaggwa MN, Gruber M, Oduor SO, Schagerl M (2013) A detailed time series assessment of the diet of lesser flamingos: further explanation for their itinerant behaviour. Hydrobiologia 710:83–93
Kambura AK, Mwirichia RK, Kasili RW, Karanja EN, Makonde HM, Boga HI (2016) Diversity of fungi in sediments and water sampled from the hot springs of Lake Magadi and little Magadi in Kenya. Afr J Microbiol Res 10:330–338
Kebede E (1997) Response of Spirulina platensis (=Arthrospira fusiformis) from Lake Chitu, Ethiopia, to salinity stress from sodium salts. J Appl Phycol 9:551–558
Kebede E, Ahlgren G (1996) Optimum growth conditions and light utilization efficiency of Spirulina platensis (= Arthrospira fusiformis) (Cyanophyta) from Lake Chitu, Ethiopia. Hydrobiologia 332:99–109
Krienitz L (2018) Lesser Flamingos. Descendants of Phoenix. Springer Nature, Berlin
Krienitz L, Kotut K (2010) Fluctuating algal food populations and the occurrence of Lesser Flamingos (Phoeniconaias minor) in three Kenyan Rift Valley lakes. J Phycol 46:1088–1096
Krienitz L, Bock C, Kotut K, Luo W (2012) Picocystis salinarum (Chlorophyta) in saline lakes and hot springs of East Africa. Phycologia 51:22–32
Krienitz L, Schagerl M (2016) Tiny and tough: Microphytes of East African soda lakes. In: Schagerl M (ed) Soda lakes of East Africa. Springer Nature, Berlin, pp 149–177
Krienitz L, Krienitz D, Dadheech PK, Hübener T, Kotut K, Luo W, Teubner K, Versfeld WD (2016a) Food algae for Lesser Flamingos: a stocktaking. Hydrobiologia 775:21–50
Krienitz L, Mähnert B, Schagerl M (2016b) Lesser Flamingo as a central element of the East African avifauna. In: Schagerl M (ed) Soda lakes of East Africa. Springer Nature, Berlin, pp 259–284
Krienitz L, Bock C, Dadheech PK, Kotut K, Luo W, Schagerl M (2016c) An underexplored resource for biotechnology: Selected microphytes of East African soda lakes and adjacent waters. In: Schagerl M (ed) Soda Lakes of East Africa. Springer Nature, Berlin, pp 323–343
Lázár D, Lengyel E, Stenger-Kovács C (2015) Nitzschia aurariae Cholnoky (Bacillariophyceae) fotoszintetikus aktivitásának vizsgálata szulfátion gradiens mentén. Hidrológiai Közlöny 95:39–41
Leatherbarrow RJ (2009) GraFit Version 7, Erithacus Software Ltd., Horley, U.K.
Leboulanger C, Agogué H, Bernard C, Bouvy M, Carré C, Cellamare M, Duval C, Fouilland E, Got P, Intertaglia L, Lavergne C, Le Floc h′ E, Roques C, Sarazin G (2017) Microbial diversity and cyanobacterial production in Dziani Dzaha crater lake, a unique tropical thalassohaline environment. PLoS One 12:e0168879
Lengyel E, Kovács AW, Padisák J, Stenger-Kovács C (2015) Photosynthetic characteristics of the benthic diatom species Nitzschia frustulum (Kützing) Grunow isolated from a soda pan along temperature-, sulfate- and chloride gradients. Aquat Ecol 49:401–416
Lengyel E, Lázár D, János A, Stenger-Kovács C (2020) Science of the total environment climate change projections for Carpathian soda pans on the basis of photosynthesis evidence from typical diatom species. Sci Total Environ 710:136241
Lewin RA, Krienitz L, Goericke R, Takeda H, Hepperle D (2000) Picocystis salinarum gen. et sp. nov. (Chlorophyta) – a new picoplanktonic green alga. Phycologia 39:560–565
Lopes dos Santos A, Gourvil P, Rodriguez-Hernandez F, Rodríguez F, Garrido JL, Vaulot D (2016) Photosynthetic pigments of oceanic Chlorophyta belonging to prasinophytes clade VII. J Phycol 52:148–155
Lopes dos Santos A, Pollina T, Gourvil P, Corre E, Marie D, Garrido JL, Francisco Rodríguez F, Mary-Hélène Noël M-H, Vaulot D, Eikrem W (2017) Chloropicophyceae, a new class of picophytoplanktonic prasinophytes. Sci Rep 7:14019
Melack JM (1988) Primary producer dynamics associated with evaporative concentration in a shallow, equatorial soda lake (Lake Elmenteita, Kenya). Hydrobiologia 158:1–14
Melack JM, Kilham P (1974) Photosynthetic rates of phytoplankton in East African alkaline, saline lakes1. Limnol Oceanogr 19:743–755
Monod J (1978) La Technique De Culture Continue Théorie Et Applications. In: Lwoff A, Ullmann (eds) Selected Papers in Molecular Biology by Jacques Monod Academic Press, NY pp 184–204
Novick A, Szilard L (1950) Experiments with the chemostat on spontaneous mutations of bacteria. Proc Natl Acad Sci 36:708–719
Nowicka-Krawczyk P, Mühlsteinová R, Hauer T (2019) Detailed characterization of the Arthrospira type species separating commercially grown taxa into the new genus Limnospira (Cyanobacteria). Sci Rep 9:36831
Nuhu AA (2013) Spirulina (Arthrospira): an important source of nutritional and medicinal compounds. J Mar Biol 2013:325636
Oduor SO, Kotut K (2016) Soda lakes of the East African rift system: the past, the present and the future. In: Schagerl M (ed) Soda lakes of East Africa. Springer Nature, Berlin, pp 365–374
Oduor SO, Schagerl M (2007) Phytoplankton primary productivity characteristics in response to photosynthetically active radiation in three Kenyan Rift Valley saline-alkaline lakes. J Plankton Res 29:1041–1050
Pálmai T, Üveges V, Krienitz L, Padisák J (2013) Az Arthrospira fusiformis és a Picocystis salinarum fotoszintézisének karakterisztikái különböző fényintenzitásokon és hőmérsékleten. Hidrológiai Közlöny 93:64–66
Pálmai T, Selmeczy GB, Szabó B, Tóth G, László, Padisák J (2016) A Microcystis flos-aquae fotoszintetikus aktivitása a Balaton keleti medencéjében 2015 nyarán (Photosynthetic activity of Microcystis flos-aquae in the eastern basin of Lake Balaton in the summer of 2015). Hidrológiai Közlöny 96:75–78
Pálmai T, Szabó B, Hubai KE, Padisák J (2018) Photosynthetic performance of two freshwater red algal species. Acta Bot Croat 77:135–140
Peduzzi P, Gruber M, Gruber M, Schagerl M (2014) The virus’s tooth - cyanophages affect the flamingo population of an African soda lake in a bottom up cascade. ISME J 8:1346–1351
Platt T, Gallegos CL, Harrison WG (1980) Photoinibition of photosynthesis in natural assemblages of marine phytoplankton. J Mar Res 38:687–701
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Rihab BA, Sabrine BO, Lina C, Hatem BO, Ali O (2017) Cadmium effect on physiological responses of the tolerant Chlorophyta specie Picocystis sp. isolated from Tunisian wastewaters. Environ Sci Pollut Res 24:1803–1810
Roesler CS, Culbertson CW, Etheridge SM, Goericke R, Kiene RP, Miller LG, Oremland RS (2002) Distribution, production, and ecophysiology of Picocystis strain ML in Mono Lake, California. Limnol Oceanogr 47:440–452
Samylina OS, Gerasimenko LM, Shadrin NV (2010) Comparative characteristic of the phototroph communities from the mineral lakes of Crimea (Ukraine) and Altai Region (Russia). Int J Algae 12:142–158
Sapozhnikov PV, Kalinina OY, Nikitin MA, Samylina OS (2016) Cenoses of phototrophic algae of ultrasaline lakes in the Kulunda steppe (Altai krai, Russian Federation). Oceanology 56:95–106
Schagerl M (ed) (2016) Soda lakes of East Africa. Springer Nature, Berlin
Schagerl M, Burian A, Gruber-Dorninger M, Odour SO, Kaggwa MN (2015) Algal communities of Kenyan soda lakes with a special focus on Arthrospira fusiformis. Fottea 15:245–257
Shafik HM, Herodek S, Voros L, Présing M, Kiss KT (1997) Growth of Cyclotella meneghiniana Kütz. 1. Effects of temperature, light and low rate of nutrient supply. Ann Limnol Int J Limnol 33:139–147
Shafik HM, Herodek S, Présing M, Vörös L (2001) Factors effecting growth and cell composition of cyanoprokaryote Cylindrospermopsis raciborskii (Wołoszyńska) Seenayya et Subba Raju. Algol Stud Hydrobiologie Suppl 103:75–93
Shafik HM, Pálmai T, Padisák J (2014) Módosított tápoldat egy trópusi sós tóból izolált Arthrospira fusiformis és Picocystis salinarum algafajok számára. Hidrológiai Közlöny 94:43–45
Shao W, Ebaid R, El-Sheekh M, Abomohra A, Eladel H (2019) Pharmaceutical applications and consequent environmental impacts of Spirulina (Arthrospira): an overview. Grasas Aceites 70:e292
Talling JF, Wood RB, Prosser MV, Baxter RM (1973) The upper limit of photosynthetic productivity by phytoplankton: evidence from Ethiopian soda lakes. Freshw Biol 3:53–76
Tarazona Delgado R, Terreros HM, Astocóndor MM, Huatuco EM (2017) Picocystis salinarum (Prasinophyceae, Chlorophyta) en las Salinas de Chilca, Lima, primer registro para el Perú. Arnaldoa 24:557–566
Torzillo G, Vonshak A (1994) Effect of light and temperature on the photosynthetic activity of the cyanobacterium Spirulina platensis. Biomass Bioenergy 6:399–403
Tuite CH (2000) The distribution and density of Lesser Flamingos in East Africa in relation to food availability and productivity. Waterbirds 23:52–63
Üveges V, Vörös L, Padisák J, Kovács AW (2011) Primary production of epipsammic algal communities in Lake Balaton (Hungary). Hydrobiologia 660:17–27
Üveges V, Tapolczai K, Krienitz L, Padisák J (2012) Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos-aquae (Cyanobacteria, Nostocaceae) winter bloom in a deep oligo-mesotrophic lake (Lake Stechlin, Germany). Hydrobiologia 698:263–272
Vareschi E (1978) The ecology of Lake Nakuru (Kenya) – I. Abundance and feeding of the Lesser Flamingo. Oecologia 32:11–35
Vareschi E (1982) The ecology of Lake Nakuru (Kenya) – III. Abiotic factors and primary production. Oecologia 55:81–101
Vonshak A, Kancharaksa N, Bunnag B, Tanticharoen M (1996) Role of light and photosynthesis on the acclimation process of the cyanobacterium Spirulina platensis to salinity stress. J Appl Phycol 8:119–124
Wang S, Lambert W, Giang S, Goericke R, Palenik B (2014) Microalgal assemblages in a poikilohaline pond. J Phycol 50:303–309
Webb WL, Newton M, Starr D (1974) Carbon dioxide exchange of Alnus rubra - a mathematical model. Oecologia 17:281–291
Wetzel RG, Likens GE (2000) Limnological analyses. Springer, New York
Williams WD (2002) Environmental threats to salt lakes and the likely status of inland saline ecosystems in 2025. Environ Conserv 29:154–167
Acknowledgments
Open access funding provided by University of Pannonia (PE). The present study is supported by the National Research Development and Innovation Office (NKFIH K120595), the European Regional Development Fund (GINOP-2.3.2-15-2016-00019), the ÚNKP-18-IV-PE-12 New National Excellence Program of the Ministry of Human Resources, and NTP-NFTÖ-16-0626 of the Ministry of Human Resources.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pálmai, T., Szabó, B., Kotut, K. et al. Ecophysiology of a successful phytoplankton competitor in the African flamingo lakes: the green alga Picocystis salinarum (Picocystophyceae). J Appl Phycol 32, 1813–1825 (2020). https://doi.org/10.1007/s10811-020-02092-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02092-6