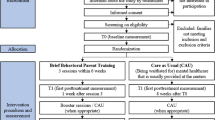
Abstract
This paper presents the rationale for a 24-week, randomized trial designed to test whether risperidone plus structured parent training would be superior to risperidone only on measures of noncompliance, irritability and adaptive functioning. In this model, medication reduces tantrums, aggression and self-injury; parent training promotes improvement in noncompliance and adaptive functioning. Thus, medication and parent training target related, but separate, outcomes. At week 24, the medication was gradually withdrawn to determine whether subjects in the combined treatment group could be managed on a lower dose or off medication without relapse. Both symptom reduction and functional improvement are important clinical treatment targets. Thus, experimental evidence on the beneficial effects of combining pharmacotherapy and exportable behavioral interventions is needed to guide clinical practice.

Similar content being viewed by others
References
Abikoff, H., Hechtman, L., Klein, R. G., Weiss, G., Fleiss, K., Etcovitch, J., et al. (2004). Symptomatic improvement in children with ADHD treated with long-term methylphenidate and multimodal psychosocial treatment. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 802–811.
Aman, M. G., Singh, N. N., Stewart, A. W., & Field, C. J. (1985). The Aberrant Behavior Checklist: A behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency, 89, 485–491.
Aman, M. G., Singh, N. N., & Turbott, S. H. (1987). Reliability of the Aberrant Behavior Checklist and the effect of variations in instructions. American Journal of Mental Deficiency, 92, 237–240.
Barkley, R. A., & Murphy, K. R. (1998). Attention deficit hyperactivity disorder: A clinical workbook (2nd ed.). New York: Guilford.
Borenstein, M., Rothman, H., & Cohen, J. (1997). Power and precision program. New Jersey: Erlbaum.
Brown, E. C., Aman, M. G., & Havercamp, S. M. (2002). Factor analysis and norms for parent ratings on the Aberrant Behavior Checklist-community for young people in special education. Research in Developmental Disabilities, 23, 45–60.
Campbell, M., Armenteros, J. L., Malone, R. P., Adams, P. B., Eisenberg, Z. W., & Overall, J. E. (1997). Neuroleptic-related dyskinesias in autistic children: a prospective, longitudinal study. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 835–843.
Carter, A. S., Volkmar, F. R., Sparrow, S. S., Wang, J. J., Lord, C., Dawson, G., et al. (1998). The Vineland Adaptive Behavior Scales: Supplementary norms for individuals with autism. Journal of Autism and Developmental Disorders, 28, 287–302.
National Research Council. (2001). Educating children with autism. Washington, DC: National Academies Press.
Fombonne, E. (2003). The prevalence of autism. Journal of the American Medical Association, 289, 87–89.
Gadow, K. D., Devincent, C. J., Pomeroy, J., & Azizian, A. (2005). Comparison of DSM-IV symptoms in elementary school-age children with PDD versus clinic and community samples. Autism, 9(4), 392–415.
Gibbons, R. D., Hedeker, D., Elkin, I., Waternaux, C., Kraemer, H. C., Greenhouse, J. B., et al. (1993). Some conceptual and statistical issues in analysis of longitudinal psychiatric data. Application to the NIMH treatment of depression collaborative research program dataset. Archives of General Psychiatry, 50, 739–750.
Guy, W. (1976). ECDEU assessment manual for psychopharmacology. Washington, DC: DHEW, NIMH.
Hollander, E., Phillips, A., Chaplin, W., Zagursky, K., Novotny, S., Wasserman, S., et al. (2005). A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology, 30, 582–589.
Johnson, C. R., Handen, B. L., Butter, E., Wagner, A., Mulick, J., Sukhodolsky, D. G., et al. (2007). Development of a parent training program for children with pervasive developmental disorders. Behavioral Interventions, 22, 201–221.
Kane, J. M., Carson, W. H., Saha, A. R., McQuade, R. D., Ingenito, G. G., Zimbroff, D. L., et al. (2002). Efficacy and safety of aripiprazole and haloperidol versus placebo in patients with schizophrenia and schizoaffective disorder. Journal of Clinical Psychiatry, 63, 763–771.
Kazdin, A., & Weisz, J. R. (Eds.). (2003). Evidence-based psychotherapies for children and adolescents. New York: Guilford Press.
Kraijer, D. (2000). Review of adaptive behavior studies in mentally retarded persons with autism/pervasive developmental disorder. Journal of Autism and Developmental Disorders, 30, 39–47.
Lord, C., Wagner, A., Rogers, S., Szatmari, P., Aman, M., Charman, T., et al. (2005). Challenges in evaluating psychosocial interventions for autistic spectrum disorders. Journal of Autism and Developmental Disorders, 35, 695–708. (discussion 709–711).
Lovaas, O. I. (1987). Behavioral treatment and normal educational and intellectual functioning in young autistic children. Journal of Consulting and Clinical Psychology, 55, 3–9.
McDougle, C. J., Scahill, L., Aman, M. G., McCracken, J. T., Tierney, E., Davies, M., et al. (2005). Risperidone for the core symptom domains of autism: results from the study by the autism network of the research units on pediatric psychopharmacology. American Journal of Psychiatry, 162, 1142–1148.
McDougle, C. J., Stigler, K. A., Erickson, C. A., & Posey, D. J. (2008). Atypical antipsychotics in children and adolescents with autistic and other pervasive developmental disorders. Journal of Clinical Psychiatry, 69(Suppl 4), 15–20.
MTA Cooperative Group. (1999). Multimodal treatment study of children with ADHD: A 14-month randomized clinical trial of treatment strategies for attention-deficit/hyperactivity disorder. Archives of General Psychiatry, 56, 1073–1086.
Pediatric OCD Treatment Study (POTS) Team. (2004). Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. Journal of the American Medical Association, 292, 1969–1976.
Research Units on Pediatric Psychopharmacology Autism Network. (2002). Risperidone in children with autism for serious behavioral problems. New England Journal of Medicine, 347, 314–321.
Research Units on Pediatric Psychopharmacology Autism Network. (2005a). Randomized, controlled, crossover trial of methylphenidate in pervasive developmental disorders with hyperactivity. Archives of General Psychiatry, 62, 1266–1274.
Research Units on Pediatric Psychopharmacology Autism Network. (2005b). Risperidone treatment of autistic disorder: Longer term benefits and blinded discontinuation after six months. American Journal of Psychiatry, 162, 1361–1369.
Research Units on Pediatric Psychopharmacology Autism Network. (2007). Parent training for children with pervasive developmental disorders: A multi-site feasibility trial. Behavioral Interventions, 22, 179–199.
Scahill, L., & Martin, A. (2005). Psychopharmacology. In F. Volkmar, A. Klin, & R. Paul (Eds.), Handbook of autism and pervasive developmental disorders (3rd ed., pp. 1102–1117). New York: Wiley.
Scahill, L., Solanto, M., & McGuire, J. (2008). The science and ethics of placebo in pediatric psychopharmacology. Ethics and Behavior, 18, 266–285.
Schreibman, L. (2000). Intensive behavioral/psychoeducational treatments for autism: Research needs and future directions. Journal of Autism and Developmental Disorders, 30, 373–378.
Shea, S., Turgay, A., Carroll, A., Schulz, M., Orlik, H., Smith, I., et al. (2004). Risperidone in the treatment of disruptive behavioral symptoms in children with autistic and other pervasive developmental disorders. Pediatrics, 114(5), 634–641.
Shrout, P. E., & Bolger, N. (2002). Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological Methods, 7, 422–445.
Smith, T., Buch, G. A., & Gamby, T. E. (2000). Parent-directed, intensive early intervention for children with pervasive developmental disorder. Research in Developmental Disabilities, 21, 297–309.
Smith, T., Scahill, L., Dawson, G., Guthrie, D., Lord, C., Odom, S., et al. (2007). Designing research studies on psychosocial interventions in autism. Journal of Autism and Developmental Disorders, 37, 354–366.
Sparrow, S. S., Balla, D. A., & Cicchetti, D. V. (1984). Vineland Adaptive Behavior scales: Survey form manual. Circle Pines: American Guidance Service.
Treatment for Adolescents with Depression Study (TADS) Team. (2004). Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. Journal of the American Medical Association, 292, 807–820.
Williams, S. K., Scahill, L., Vitiello, B., Aman, M. G., Arnold, L. E., McDougle, C. J., et al. (2006). Risperidone and adaptive behavior in children with autism. Journal of the American Academy of Child and Adolescent Psychiatry, 45, 431–439.
Acknowledgments
This study was supported by the following cooperative agreement grants from the National Institute of Mental Health: U10MH66768 (P.I., M. Aman), U10MH66766 (P.I., C. McDougle), and U10MH66764 (P.I., L. Scahill). The views expressed in this article are those of the authors and do not necessarily reflect the official position of the National Institute of Mental Health, the National Institutes of Health, or any other part of the U.S. Department of Health and Human Services. The authors would like to acknowledge the following people: Stacie Trollinger, Patricia Shugarts, Allison Gavaletz, Kristy Hall, Arlene Kohn, Kathy Koenig, MSN, Maryellen Pachler, MSN, Sue Thompson, MSN, Roumen Niklov, MD, Kristina Siefker, MS, Mariana Zaphiriou, Erin Kustan, Scientific Advisors, Data Safety and Monitoring Board.
Disclosure
Dr. Scahill consults to Janssen Pharmaceutica, Supernus and Bristol-Myers Squibb, Neuropharm, Shire. Dr. Aman consults to Janssen Pharmaceutica, Eli Lilly, Forest Labs and Abbott. Dr. Arnold consults to Eli Lilly, McNeil, Novartis, Noven, Shire, Sigma Tau and Targacept. Dr. McDougle consults to Forest Research Institute, Bristol-Myers Squibb, Eli Lilly, Janssen Pharmaceutica. Dr. McCracken consults to Janssen Pharmaceutica, Eli Lilly, Abbott, Bristol Myers Squibb, Shire, Wyeth, Pfizer, Cephalon, and McNeil. Dr. Handen consults to Bristol Myers Squibb, Forest Labs, Pfizer. The other authors have no financial relationships with for-profit enterprises to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
From the Research Units on Pediatric Psychopharmacology (RUPP) Autism Network.
Rights and permissions
About this article
Cite this article
Scahill, L., Aman, M.G., McDougle, C.J. et al. Trial Design Challenges When Combining Medication and Parent Training in Children with Pervasive Developmental Disorders. J Autism Dev Disord 39, 720–729 (2009). https://doi.org/10.1007/s10803-008-0675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10803-008-0675-2