Abstract
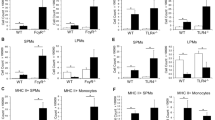
The tumour microenvironment predominantly consists of macrophages with phenotypes ranging from pro-inflammatory (M1-like) to anti-inflammatory (M2-like). Trehalose-6,6′-dibehenate (TDB) displays moderate anti-tumour activity and stimulates M1-like macrophages via the macrophage inducible C-type lectin (Mincle) resulting in IL-1β production. In this study, we examined if monosodium urate (MSU), a known vaccine adjuvant, can boost IL-1β production by TDB-stimulated macrophages. We investigated the effect of MSU/TDB co-treatment on IL-1β production by GM-CSF (M1-like) and M-CSF/IL-4 (M2-like) differentiated mouse bone marrow macrophages (BMMs) and found that MSU/TDB co-treatment of GM-CSF BMMs significantly enhanced IL-1β production in a Mincle-dependent manner. Western blot analysis showed that increased IL-1β production by GM-CSF BMMs was associated with the induction of pro-IL-1β expression by TDB rather than MSU. Flow cytometry analysis showed that MSU/TDB co-stimulation of GM-CSF BMMs led to greater expansion of CD86high/MHC IIhigh and CD86low/MHC IIlow subpopulations; however, only the latter showed increased production of IL-1β. Together, these findings provide evidence of the potential to use MSU/TDB co-treatment to boost IL-1β-mediated anti-tumour activity in M1-like tumour-associated macrophages.



Similar content being viewed by others
Abbreviations
- BMMs:
-
Bone marrow macrophages
- GM-CSF:
-
Granulocyte macrophage-colony stimulating factor
- M-CSF/IL-4:
-
Macrophage-colony stimulating factor/interleukin-4
- MSU:
-
Monosodium urate
- TDB:
-
Trehalose dibehenate
References
Temizoz, B., E. Kuroda, and K.J. Ishii. 2016. Vaccine adjuvants as potential cancer immunotherapeutics. International Immunology 28: 329–338.
Bowen, W.J., K.S. Abhishek, L. Batra, H. Barsoumian, and H. Shirwan. 2018. Current challenges for cancer vaccine adjuvant development. Expert Review of Vaccines 17: 207–215.
Pasquale, A.D., S. Preiss, F.T. Da Silva, and N. Garcon. 2015. Vaccine adjuvants: From 1920 to 2015 and beyond. Vaccines 3: 320–343.
Zepp, F. 2016. Principles of vaccination. Methods in Molecular Biology 1403: 57–84.
van Ravenswaay Claasen, H.H., P.M. Kluin, and G.J. Fleuren. 1992. Tumor infiltrating cells in human cancer. On the possible role of CD16+ macrophages in antitumor cytotoxicity. Laboratory Investigation 67: 166–174.
Mosser, D.M., and J.P. Edwards. 2008. Exploring the full spectrum of macrophage activation. Nature Reviews. Immunology 8: 958–969.
Mantovani, A., F. Marchesi, A. Malesci, L. Laghi, and P. Allavena. 2017. Tumour-associated macrophages as treatment targets in oncology. Nature Reviews Clinical Oncology 14: 399–416.
Buhtoiarov, I.N., P.M. Sondel, J.M. Wigginton, T.N. Buhtoiarova, E.M. Yanke, D.A. Mahvi, and A.L. Rakhmilevich. 2011. Anti-tumour synergy of cytotoxic chemotherapy and anti-CD40 plus CpG-ODN immunotherapy through repolarization of tumour-associated macrophages. Immunology 132: 226–239.
Shi, Y., M.A.R. Felder, P.M. Sondel, and A.L. Rakhmilevich. 2015. Synergy of anti-CD40, CpG and MPL in activation of mouse macrophages. Molecular Immunology 66: 208–215.
Dewan, M.Z., C. Vanpouille-Box, N. Kawashima, S. DiNapoli, J.S. Babb, S.C. Formenti, S. Adams, and S. Demaria. 2012. Synergy of topical toll-like receptor 7 agonist with radiation and low-dose cyclophosphamide in a mouse model of cutaneous breast cancer. Clinical Cancer Research 18: 6668–6678.
Hussain, S.F., L.-Y. Kong, J. Jordan, C. Conrad, T. Madden, I. Fokt, W. Priebe, and A.B. Heimberger. 2007. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Research 67: 9630–9636.
Edwards, J.P., and L.A. Emens. 2010. The multikinase inhibitor Sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE2 in murine macrophages. International Immunopharmacology 10: 1220–1228.
Bloch, H., and H. Noll. 1954. Studies on the virulence of tubercle bacilli; the effect of cord factor on murine tuberculosis. British Journal of Experimental Pathology 36: 8–17.
Ishikawa, E., T. Ishikawa, Y.S. Morita, K. Toyonaga, H. Yamada, O. Takeuchi, T. Kinoshita, S. Akira, Y. Yoshikai, and S. Yamasaki. 2009. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. The Journal of Experimental Medicine 206: 2879–2888.
Schoenen, H., B. Bodendorfer, K. Hitchens, S. Manzanero, K. Werninghaus, F. Nimmerjahn, E.M. Agger, S. Stenger, P. Andersen, J. Ruland, G.D. Brown, C. Wells, and R. Lang. 2010. Cutting edge: Mincle is essential for recognition and Adjuvanticity of the mycobacterial cord factor and its synthetic analog Trehalose-Dibehenate. Journal of Immunology 184: 2756–2760.
Braganza, C., T. Teunissen, M.S.M. Timmer, and B. Stocker. 2018. Synthetic Mincle ligands. Frontiers in Immunology 8: 1940.
Yarkoni, E., L. Wang, and A. Bekierkunst. 1974. Suppression of growth of Ehrlich ascites tumor cells in mice by trehalose-6,6′-dimycolate (cord factor) and BCG. Infection and Immunity 9: 977–984.
Yarkoni, E., E. Lederer, and H.J. Rapp. 1981. Immunotherapy of experimental cancer with a mixture of synthetic muramyl dipeptide and trehalose dimycolate. Infection and Immunity 32: 273–276.
Watanabe, R., Y.C. Yoo, K. Hata, M. Mitobe, Y. Koike, M. Nishizawa, D.M. Garcia, Y. Nobuchi, H. Imagawa, H. Yamada, and I. Azuma. 1999. Inhibitory effect of trehalose dimycolate (TDM) and its stereoisometric derivatives, trehalose dicorynomycolates (TDCMs), with low toxicity on lung metastasis of tumour cells in mice. Vaccine 17: 1484–1492.
Yamamoto, H., M. Oda, M. Nakano, N. Watanabe, K. Yabiku, M. Shibutani, M. Inoue, H. Imagawa, M. Nagahama, S. Himeno, K. Setsu, J. Sakurai, and M. Nishizawa. 2013. Development of Vizantin, a safe immunostimulant, based on the structure-activity relationship of trehalose-6,6′-dicorynomycolate. Journal of Medicinal Chemistry 56: 381–385.
Pimm, M.V., R.W. Baldwin, J. Polonsky, and E. Lederer. 1979. Immunotherapy of an ascitic rat hepatoma with cord factor (trehalose-6,6′-dimycolate) and synthetic analogues. International Journal of Cancer 24: 780–785.
Nishikawa, Y., T. Katori, K. Kukita, and T. Ikekawa. 1982. Synthesis and anti-tumour effects of 6,6′-di-O-acyl-α,α'-trehaloses. Nippon Kagaku Kaishi 10: 1661–1666.
Kodar, K., J.L. Harper, M.J. McConnell, M.S.M. Timmer, and B.L. Stocker. 2017. The Mincle ligand trehalose dibehenate differentially modulates M1-like and M2-like macrophage phenotype and function via Syk signaling. Immunity, Inflammation and Disease 5: 503–514.
Werninghaus, K., A. Babiak, O. Groß, C. Hölscher, H. Dietrich, E.M. Agger, J. Mages, A. Mocsai, H. Schoenen, K. Finger, F. Nimmerjahn, G.D. Brown, C. Kirschning, A. Heit, P. Andersen, H. Wagner, J. Ruland, and R. Lang. 2009. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ–Syk–Card9–dependent innate immune activation. The Journal of Experimental Medicine 206: 89–97.
Schweneker, K., O. Gorka, M. Schweneker, H. Poeck, J. Tschopp, C. Peschel, J. Ruland, and O. Groß. 2013. The mycobacterial cord factor adjuvant analogue trehalose-6,6′-dibehenate (TDB) activates the Nlrp3 inflammasome. Immunobiology 218: 664–673.
Giamarellos-Bourboulis, E.J., M. Mouktaroudi, E. Bodar, J. Van Der Ven, B.J. Kullberg, M.G. Netea, et al. 2009. Crystals of monosodium urate monohydrate enhance lipopolysaccharide-induced release of interleukin 1 βby mononuclear cells through a caspase 1-mediated process. Annals of the Rheumatic Diseases 68: 273–278.
Chen, C.J., Y. Shi, A. Hearn, K. Fitzgerald, D. Golenbock, G. Reed, S. Akira, and K.L. Rock. 2006. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. The Journal of Clinical Investigation 116: 2262–2271.
Taus, F., M.B. Santucci, E. Greco, M. Morandi, I. Palucci, S. Mariotti, et al. 2015. Monosodium urate crystals promote innate anti-mycobacterial immunity and improve BCG efficacy as a vaccine against tuberculosis. PLoS One 10: 1–16.
Kuhn, S., E.J. Hyde, J. Yang, F.J. Rich, J.L. Harper, J.R. Kirman, and F. Ronchese. 2013. Increased numbers of monocyte-derived dendritic cells during successful tumor immunotherapy with immune-activating agents. Journal of Immunology 191: 1984–1992.
Shi, Y., J.E. Evans, and K.L. Rock. 2003. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425: 516–521.
Hu, D.-E., A.M. Moore, L.L. Thomsen, and K.M. Brindle. 2004. Uric acid promotes tumor immune rejection. Cancer Research 64: 5059–5062.
Dziaman, T., Z. Banaszkiewicz, K. Roszkowski, D. Gackowski, E. Wisniewska, R. Rozalski, M. Foksinski, A. Siomek, E. Speina, A. Winczura, A. Marszalek, B. Tudek, and R. Olinski. 2014. 8-Oxo-7,8-dihydroguanine and uric acid as efficient predictors of survival in colon cancer patients. International Journal of Cancer 134: 376–383.
Slobodnick, A., S. Krasnokutsky, R.A. Lehmann, R.T. Keenan, J. Quach, F. Francois, and M.H. Pillinger. 2018. Colorectal Cancer among gout patients undergoing colonoscopy. Journal of Clinical Rheumatology: 1. https://doi.org/10.1097/RHU.0000000000000893.
Fleetwood, A.J., T. Lawrence, J.A. Hamilton, and A.D. Cook. 2007. Granulocyte-macrophage Colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: Implications for CSF blockade in inflammation. Journal of Immunology 178: 5245–5252.
Hamilton, T.A., C. Zhao, P.G. Pavicic, and S. Datta. 2014. Myeloid colony-stimulating factors as regulators of macrophage polarization. Frontiers in Immunology 5: 1–6.
Khan, A.A., S.H. Chee, R.J. McLaughlin, J.L. Harper, F. Kamena, M.S. Timmer, and B.L. Stocker. 2011. Long-chain lipids are required for the innate immune recognition of trehalose diesters by macrophages. Chembiochem 12: 2572–2576.
Martin, W.J., M. Walton, and J.L. Harper. 2009. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis and Rheumatism 60: 281–289.
Haabeth, O.A.W., K.B. Lorvik, H. Yagita, B. Bogen, and A. Corthay. 2016. Interleukin-1 is required for cancer eradication mediated by tumor-specific Th1 cells. Oncoimmunology 5: e1039763.
He, Y., H. Hara, and G. Núñez. 2016. Mechanism and regulation of NLRP3 Inflammasome activation. Trends in Biochemical Sciences 41: 1012–1021.
Helft, J., J. Böttcher, P. Chakravarty, S. Zelenay, J. Huotari, B.U. Schraml, D. Goubau, and C. Reise Sousa. 2015. GM-CSF mouse bone marrow cultures comprise a heterogeneous population of CD11c(+)MHCII(+) macrophages and dendritic cells. Immunity 42: 1197–1211.
Na, Y.R., D. Jung, G.J. Gu, and S.H. Seok. 2016. GM-CSF grown bone marrow derived cells are composed of phenotypically different dendritic cells and macrophages. Molecules and Cells 39: 734–741.
Guermonprez, P., J. Valladeau, L. Zitvogel, C. Théry, and S. Amigorena. 2002. Antigen presentaion and T cell stimulation by dendritic cells. Annual Review of Immunology 20: 621–667.
Wang, C., X. Yu, Q. Cao, Y. Wang, G. Zheng, T.K. Tan, et al. 2013. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunology 14: 1–10.
Acknowledgments
We thank Professor Sho Yamasaki for kindly providing the Mincle−/− mice.
Funding
This work was supported by the Cancer Society of New Zealand (2016/25) and the Health Research Council of New Zealand (Hercus Fellowship, BLS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
All experimental procedures were approved by the Victoria University Animal Ethics Committee in accordance with their guidelines for the care of animals (protocol nr 22371).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wahi, K., Kodar, K., McConnell, M.J. et al. MSU Crystals Enhance TDB-Mediated Inflammatory Macrophage IL-1β Secretion. Inflammation 42, 1129–1136 (2019). https://doi.org/10.1007/s10753-019-00976-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-019-00976-5