Abstract
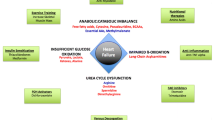
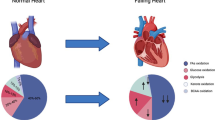
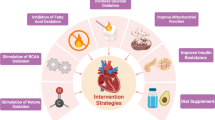
Heart failure (HF) is a clinical syndrome caused by a decline in cardiac systolic or diastolic function, which leaves the heart unable to pump enough blood to meet the normal physiological requirements of the human body. It is a serious disease burden worldwide affecting nearly 23 million patients. The concept that heart failure is “an engine out of fuel” has been generally accepted and metabolic remodeling has been recognized as an important aspect of this condition; it is characterized by defects in energy production and changes in metabolic pathways involved in the regulation of essential cellular functions such as the process of substrate utilization, the tricarboxylic acid cycle, oxidative phosphorylation, and high-energy phosphate metabolism. Advances in second-generation sequencing, proteomics, and metabolomics have made it possible to perform comprehensive tests on genes and metabolites that are crucial in the process of HF, thereby providing a clearer and comprehensive understanding of metabolic remodeling during HF. In recent years, new metabolic changes such as ketone bodies and branched-chain amino acids were demonstrated as alternative substrates in end-stage HF. This systematic review focuses on changes in metabolic substrate utilization during the progression of HF and the underlying regulatory mechanisms. Accordingly, the conventional concepts of metabolic remodeling characteristics are reviewed, and the latest developments, particularly multi-omics studies, are compiled.


Similar content being viewed by others
References
Shen L et al (2017) Declining risk of sudden death in heart failure. N Engl J Med 377(1):41–51
Dunlay SM, Roger VL, Redfield MM (2017) Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14(10):591–602
Velazquez EJ et al (2016) Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 374(16):1511–1520
Filion KB et al (2016) A multicenter observational study of incretin-based drugs and heart failure. N Engl J Med 374(12):1145–1154
Felker GM et al (2017) Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA 318(8):713–720
Ho KK et al (1993) The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol 22(4 Suppl A):6A–13A
Doenst T, Nguyen TD, Abel ED (2013) Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res 113(6):709–724
Byrne NJ et al (2016) Normalization of cardiac substrate utilization and left ventricular hypertrophy precede functional recovery in heart failure regression. Cardiovasc Res 110(2):249–257
Neubauer S (2007) The failing heart—an engine out of fuel. N Engl J Med 356(11):1140–1151
Stanley WC, Recchia FA, Lopaschuk GD (2005) Myocardial substrate metabolism in the normal and failing heart. Physiol Rev 85(3):1093–1129
Fillmore N, Lopaschuk GD (2013) Targeting mitochondrial oxidative metabolism as an approach to treat heart failure. Biochim Biophys Acta 1833(4):857–865
Lopaschuk GD et al (2010) Myocardial fatty acid metabolism in health and disease. Physiol Rev 90(1):207–258
Szablewski L (2017) Glucose transporters in healthy heart and in cardiac disease. Int J Cardiol 230(1):70–75
Liu LX et al (2017) PDK4 inhibits cardiac pyruvate oxidation in late pregnancy. Circ Res 121(12):1370–1378
Vimercati C et al (2014) Beneficial effects of acute inhibition of the oxidative pentose phosphate pathway in the failing heart. Am J Physiol Heart Circ Physiol 306(5):H709–H717
Schulze PC, Drosatos K, Goldberg IJ (2016) Lipid use and misuse by the heart. Circ Res 118(11):1736–1751
Carley AN, Lewandowski ED (2016) Triacylglycerol turnover in the failing heart. Biochim Biophys Acta 1861(10):1492–1499
Jenei ZA et al (2011) Packing of transmembrane domain 2 of carnitine palmitoyltransferase-1A affects oligomerization and malonyl-CoA sensitivity of the mitochondrial outer membrane protein. FASEB J 25(12):4522–4530
Abdurrachim D et al (2015) Good and bad consequences of altered fatty acid metabolism in heart failure: evidence from mouse models. Cardiovasc Res 106(2):194–205
O’Neill HM et al (2014) AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia 57(8):1693–1702
Saha AK et al (2000) Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J Biol Chem 275(32):24279–24283
Barreto-Torres G et al (2015) The beneficial effects of AMP kinase activation against oxidative stress are associated with prevention of PPARalpha-cyclophilin D interaction in cardiomyocytes. Am J Physiol Heart Circ Physiol 308(7):H749–H758
Sung MM et al (2015) AMPK deficiency in cardiac muscle results in dilated cardiomyopathy in the absence of changes in energy metabolism. Cardiovasc Res 107(2):235–245
Ashrafian H, Frenneaux MP, Opie LH (2007) Metabolic mechanisms in heart failure. Circulation 116(4):434–448
Abushouk AI et al (2017) Peroxisome proliferator-activated receptors as therapeutic targets for heart failure. Biomed Pharmacother 95(1):692–700
Lam VH et al (2015) Activating PPARalpha prevents post-ischemic contractile dysfunction in hypertrophied neonatal hearts. Circ Res 117(1):41–51
Smeets PJ et al (2008) Cardiac hypertrophy is enhanced in PPAR alpha−/− mice in response to chronic pressure overload. Cardiovasc Res 78(1):79–89
Drosatos K et al (2016) Cardiac myocyte KLF5 regulates Ppara expression and cardiac function. Circ Res 118(2):241–253
Palomer X et al (2016) PPARbeta/delta and lipid metabolism in the heart. Biochim Biophys Acta 1861(10):1569–1578
Burkart EM et al (2007) Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest 117(12):3930–3939
Riehle C, Abel ED (2012) PGC-1 proteins and heart failure. Trends Cardiovasc Med 22(4):98–105
Abo AO, Lopaschuk GD (2014) Role of CoA and acetyl-CoA in regulating cardiac fatty acid and glucose oxidation. Biochem Soc Trans 42(4):1043–1051
Guo Z (2015) Pyruvate dehydrogenase, Randle cycle, and skeletal muscle insulin resistance. Proc Natl Acad Sci U S A 112(22):E2854
Gomez-Arroyo J et al (2013) Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circ Heart Fail 6(1):136–144
Christe ME, Rodgers RL (1994) Altered glucose and fatty acid oxidation in hearts of the spontaneously hypertensive rat. J Mol Cell Cardiol 26(10):1371–1375
Massie BM et al (1995) Myocardial high-energy phosphate and substrate metabolism in swine with moderate left ventricular hypertrophy. Circulation 91(6):1814–1823
Degens H et al (2006) Cardiac fatty acid metabolism is preserved in the compensated hypertrophic rat heart. Basic Res Cardiol 101(1):17–26
Seymour AM et al (2015) In vivo assessment of cardiac metabolism and function in the abdominal aortic banding model of compensated cardiac hypertrophy. Cardiovasc Res 106(2):249–260
Kato T et al (2010) Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Circ Heart Fail 3(3):420–430
O’Donnell JM et al (2008) The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol 44(2):315–322
Lai L et al (2014) Energy metabolic reprogramming in the hypertrophied and early stage failing heart: a multisystems approach. Circ Heart Fail 7(6):1022–1031
Burke MA et al (2016) Molecular profiling of dilated cardiomyopathy that progresses to heart failure. JCI Insight 1(6):e86898
Lionetti V, Stanley WC, Recchia FA (2011) Modulating fatty acid oxidation in heart failure. Cardiovasc Res 90(2):202–209
Heggermont WA et al (2016) Metabolic support for the heart: complementary therapy for heart failure? Eur J Heart Fail 18(12):1420–1429
Pereira RO et al (2014) GLUT1 deficiency in cardiomyocytes does not accelerate the transition from compensated hypertrophy to heart failure. J Mol Cell Cardiol 72(1):95–103
Yan J et al (2009) Increased glucose uptake and oxidation in mouse hearts prevent high fatty acid oxidation but cause cardiac dysfunction in diet-induced obesity. Circulation 119(21):2818–2828
Kundu BK et al (2015) Remodeling of glucose metabolism precedes pressure overload-induced left ventricular hypertrophy: review of a hypothesis. Cardiology 130(4):211–220
Bedi KJ et al (2016) Evidence for Intramyocardial disruption of lipid metabolism and increased myocardial ketone utilization in advanced human heart failure. Circulation 133(8):706–716
Peterzan MA et al (2017) Metabolic remodeling in hypertrophied and failing myocardium: a review. Am J Physiol Heart Circ Physiol 313(3):H597–H616
El AZ et al (1992) Fatty acid oxidation and mechanical performance of volume-overloaded rat hearts. Am J Phys 262(4 Pt 2):H1068–H1074
Pound KM et al (2009) Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res 104(6):805–812
Lei B et al (2004) Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J Mol Cell Cardiol 36(4):567–576
Sansbury BE et al (2014) Metabolomic analysis of pressure-overloaded and infarcted mouse hearts. Circ Heart Fail 7(4):634–642
Davila-Roman VG et al (2002) Altered myocardial fatty acid and glucose metabolism in idiopathic dilated cardiomyopathy. J Am Coll Cardiol 40(2):271–277
Zhabyeyev P et al (2013) Pressure-overload-induced heart failure induces a selective reduction in glucose oxidation at physiological afterload. Cardiovasc Res 97(4):676–685
Amorim PA et al (2010) Myocardial infarction in rats causes partial impairment in insulin response associated with reduced fatty acid oxidation and mitochondrial gene expression. J Thorac Cardiovasc Surg 140(5):1160–1167
Osorio JC et al (2002) Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Circulation 106(5):606–612
Doenst T et al (2010) Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Cardiovasc Res 86(3):461–470
Gupte AA et al (2014) Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet 7(3):266–276
Tuunanen H, Ukkonen H, Knuuti J (2008) Myocardial fatty acid metabolism and cardiac performance in heart failure. Curr Cardiol Rep 10(2):142–148
Riehle C, Abel ED (2016) Insulin signaling and heart failure. Circ Res 118(7):1151–1169
Aubert G et al (2016) The failing heart relies on ketone bodies as a fuel. Circulation 133(8):698–705
Sun H et al (2016) Catabolic defect of branched-chain amino acids promotes heart failure. Circulation 133(21):2038–2049
Foster DB et al (2016) Integrated omic analysis of a guinea pig model of heart failure and sudden cardiac death. J Proteome Res 15(9):3009–3028
Hunter WG et al (2016) Metabolomic profiling identifies novel circulating biomarkers of mitochondrial dysfunction differentially elevated in heart failure with preserved versus reduced ejection fraction: evidence for shared metabolic impairments in clinical heart failure. J Am Heart Assoc 5(8):e003190
Ruiz M et al (2017) Circulating acylcarnitine profile in human heart failure: a surrogate of fatty acid metabolic dysregulation in mitochondria and beyond. Am J Physiol Heart Circ Physiol 313(4):H768–H781
Ahmad T et al (2016) Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J Am Coll Cardiol 67(3):291–299
Fragasso G et al (2006) A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. J Am Coll Cardiol 48(5):992–998
Fragasso G et al (2011) Effect of partial inhibition of fatty acid oxidation by trimetazidine on whole body energy metabolism in patients with chronic heart failure. Heart 97(18):1495–1500
Tuunanen H et al (2006) Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation 114(20):2130–2137
Salerno A et al (2015) Effects of short-term manipulation of serum FFA concentrations on left ventricular energy metabolism and function in patients with heart failure: no association with circulating bio-markers of inflammation. Acta Diabetol 52(4):753–761
Martin MA et al (2000) Myocardial carnitine and carnitine palmitoyltransferase deficiencies in patients with severe heart failure. Biochim Biophys Acta 1502(3):330–336
Fillmore N, Mori J, Lopaschuk GD (2014) Mitochondrial fatty acid oxidation alterations in heart failure, ischaemic heart disease and diabetic cardiomyopathy. Br J Pharmacol 171(8):2080–2090
Wang Y et al (2013) Integrated proteomic and metabolomic analysis reveals the NADH-mediated TCA cycle and energy metabolism disorders based on a new model of chronic progressive heart failure. Mol BioSyst 9(12):3135–3145
Warren JS et al (2017) Metabolic reprogramming via PPARalpha signaling in cardiac hypertrophy and failure: from metabolomics to epigenetics. Am J Physiol Heart Circ Physiol 313(3):H584–H596
Kaimoto S et al (2017) Activation of PPAR-alpha in the early stage of heart failure maintained myocardial function and energetics in pressure-overload heart failure. Am J Physiol Heart Circ Physiol 312(2):H305–H313
Oka S et al (2015) Peroxisome proliferator activated receptor-alpha association with silent information regulator 1 suppresses cardiac fatty acid metabolism in the failing heart. Circ Heart Fail 8(6):1123–1132
Morgan EE et al (2006) Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol 290(5):H1899–H1904
Ogata T et al (2004) Myocardial fibrosis and diastolic dysfunction in deoxycorticosterone acetate-salt hypertensive rats is ameliorated by the peroxisome proliferator-activated receptor-alpha activator fenofibrate, partly by suppressing inflammatory responses associated with the nuclear factor-kappa-B pathway. J Am Coll Cardiol 43(8):1481–1488
Brigadeau F et al (2007) The PPARalpha activator fenofibrate slows down the progression of the left ventricular dysfunction in porcine tachycardia-induced cardiomyopathy. J Cardiovasc Pharmacol 49(6):408–415
Cheng L et al (2004) Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med 10(11):1245–1250
El AH et al (2013) The hypoxia-inducible microRNA cluster miR-199a approximately 214 targets myocardial PPARdelta and impairs mitochondrial fatty acid oxidation. Cell Metab 18(3):341–354
Sihag S et al (2009) PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol 46(2):201–212
Riehle C et al (2011) PGC-1beta deficiency accelerates the transition to heart failure in pressure overload hypertrophy. Circ Res 109(7):783–793
Lopaschuk GD, Ussher JR (2016) Evolving concepts of myocardial energy metabolism: more than just fats and carbohydrates. Circ Res 119(11):1173–1176
Wende AR et al (2017) Metabolic origins of heart failure. JACC Basic Transl Sci 2(3):297–310
Cotter DG, Schugar RC, Crawford PA (2013) Ketone body metabolism and cardiovascular disease. Am J Physiol Heart Circ Physiol 304(8):H1060–H1076
Yokokawa T et al (2016) Exhaled acetone concentration is related to hemodynamic severity in patients with non-ischemic chronic heart failure. Circ J 80(5):1178–1186
Obokata M et al (2017) Association between circulating ketone bodies and worse outcomes in hemodialysis patients. J Am Heart Assoc 6(10):e006885
Taegtmeyer H (2016) Failing heart and starving brain: ketone bodies to the rescue. Circulation 134(4):265–266
Kolwicz SJ, Airhart S, Tian R (2016) Ketones step to the plate: a game changer for metabolic remodeling in heart failure? Circulation 133(8):689–691
Wang W et al (2016) Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 311(5):H1160–H1169
Tanada Y et al (2015) Branched-chain amino acids ameliorate heart failure with cardiac cachexia in rats. Life Sci 137(1):20–27
Takata M et al (2017) An exploratory study on the efficacy and safety of a BCAA preparation used in combination with cardiac rehabilitation for patients with chronic heart failure. BMC Cardiovasc Disord 17(1):205
Huynh K (2016) Heart failure: ketone bodies as fuel in heart failure. Nat Rev Cardiol 13(3):122–123
Biesele JJ, Tobioka M (1956) Mitochondria in living cells: an analysis of movements. J Biophys Biochem Cytol 2(4 Suppl):319–324
Maneechote C et al (2017) Roles of mitochondrial dynamics modulators in cardiac ischaemia/reperfusion injury. J Cell Mol Med 21(11):2643–2653
Nan J et al (2017) TNFR2 stimulation promotes mitochondrial fusion via Stat3- and NF-kB-dependent activation of OPA1 expression. Circ Res 121(4):392–410
Wai T et al (2015) Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science 350(6265):aad0116
Nan J et al (2017) Molecular regulation of mitochondrial dynamics in cardiac disease. Biochim Biophys Acta 1864(7):1260–1273
Martin OJ et al (2014) A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circ Res 114(4):626–636
Tsushima K et al (2018) Mitochondrial reactive oxygen species in Lipotoxic hearts induce post-translational modifications of AKAP121, DRP1, and OPA1 that promote mitochondrial fission. Circ Res 122(1):58–73
Hensley CT et al (2016) Metabolic heterogeneity in human lung tumors. Cell 164(4):681–694
Faubert B et al (2017) Lactate metabolism in human lung tumors. Cell 171(2):358–371.e9
Li Q et al (2015) Multiple mass isotopomer tracing of acetyl-CoA metabolism in Langendorff-perfused rat hearts: channeling of acetyl-CoA from pyruvate dehydrogenase to carnitine acetyltransferase. J Biol Chem 290(13):8121–8132
Nadtochiy SM et al (2015) Metabolomic profiling of the heart during acute ischemic preconditioning reveals a role for SIRT1 in rapid cardioprotective metabolic adaptation. J Mol Cell Cardiol 88(1):64–72
Funding
This study was funded by CAMS Innovation Fund for Medical Sciences (2016-I2M-1-015) and the National Natural Science Foundation of China (81670376).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Chen, L., Song, J. & Hu, S. Metabolic remodeling of substrate utilization during heart failure progression. Heart Fail Rev 24, 143–154 (2019). https://doi.org/10.1007/s10741-018-9713-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-018-9713-0