Abstract
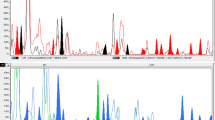
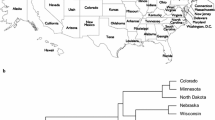
The ‘Morelos’ accessions of Amaranthus from Mexico demonstrate taxonomic ambiguity at the basic morphologic level. The main cause is the enormous morphological and genetic variation exhibited by the species in the genus. Although basic morphological criteria can be applied to herbarium specimens or germplasm collections for quick taxonomic identification, the morphological data alone can be misleading. To ascertain the taxonomic identity of the ‘Morelos’ accessions and their hypothesized species affiliation to Amaranthus caudatus or Amaranthus cruentus, we conducted a comparative analysis of phylogenetic relationships among these taxa/accessions using amplified fragment length polymorphism (AFLP) and micromorphology methods. Based on AFLP data, all the controversial ‘Morelos’ accessions can be consistently placed into a single A. cruentus species clade, which is clearly separated from the A. caudatus species clade. The AFLP-based phylogenetic relationship of ‘Morelos’ and delimitation of A. cruentus and A. caudatus are further supported by micromorphology, showing that the combination of these techniques can provide more reliable data for germplasm identification than each method used alone.
Similar content being viewed by others
Abbreviations
- AFLP:
-
amplified fragment-length polymorphism
- RAPD:
-
randomly amplified polymorphic DNA
- RFLP:
-
restriction fragment length polymorphism
- SEM:
-
scanning electron microscopy
References
Brenner D.M., Baltensperger D.D., Kulakow P.A., Lehmann J.W., Myers R.L., Slabbert M.M. and Sleugh B.B. (2000). Genetic resources and breeding of Amaranthus. Plant Breed. Rev. 19: 227–285 .
Busso C.S., Devos K.M., Ross G., Mortimore M., Adams W.M., Ambrose M.J., Alldrick S. and Gale M.D. (2000). Genetic diversity within and among landraces of pearl millet (Pennisetum glaucum) under farmer management in West Africa. Genet. Resour. Crop Evol. 47: 561–568 .
Cai Y., Sun M. and Corke H. (1998). Colorant properties and stability of Amaranthus betacyanin pigments. J. Agric. Food. Chem. 46: 4491–4495 .
Chan K.F. and Sun M. (1997). Genetic diversity detected by isozyme and RAPD analysis of crop and wild species of Amaranthus. Theor. Appl. Genet. 95: 865–873 .
Cheng Z., Lu B.-R., Sameshima K., Fu D.-X. and Chen J.-K. (2004). Identification and genetic relationships of kenaf (Hibiscus cannabinus L.) germplasm revealed by AFLP analysis. Genet. Resour. Crop Evol. 51: 393–401 .
Costea M., Sanders A. and Waines G. (2001). Preliminary results toward a revision of the Amaranthus hybridus species complex (Amaranthaceae). Sida 19: 931–974 .
Dehmer K.J. and Hammer K. (2004). Taxonomic status and geographic provenance of germplasm accessions in the Solanum nigrum L. complex: AFLP data. Genet. Resour. Crop Evol. 51: 551–558 .
Hansen L.B., Siegismund H.R. and Jørgensen R.B. (2001). Introgression between oilseed rape (Brassica napus L.) and its weedy relative B. rapa L. in a natural population. Genet. Resour. Crop Evol. 48: 621–627 .
Huang J. and Sun M. (1999). A modified AFLP with fluorescence-labeled primers and automated DNA sequencer detection for efficient fingerprinting analysis in plants. Biotechnol. Tech. 13: 277–278 .
Huang J., Corke H. and Sun M. (2002). Highly polymorphic AFLP markers as a complementary tool to ITS sequences in assessing genetic diversity and phylogenetic relationships of sweetpotato (Ipomoea batatas (L.) Lam.) and its wild relatives. Genet. Resour. Crop Evol. 49: 541–550 .
Jones C.J., Edwards K.J., Castaglione S., Winfield M.O., Sale F., Bredemeijer G., Vosman B., Matthes M., Daly A., Brettschneider R., Bettini P., Buiatti P., Maestri E., Malcevshi A., Marmiroli N., Aert R., Volckaert G., Rueda J., Linacero R., Vazquez A., Karp A. and Wiel C. (1997). Reproducibility of RAPD, AFLP and SSR markers in plants by a network of European laboratories. Mol. Breed. 3: 381–390 .
Lanoue K.Z., Wolf P.G., Browning S. and Hood E.E. (1996). Phylogenetic analysis of restriction-site variation in wild and cultivated Amaranthus species (Amaranthaceae). Theor. Appl. Genet. 93: 722–732 .
Lin J.J., Kuo J., Ma J., Saunders J.A., Beard H.S., MacDonald M.H., Kenworthy W., Ude G.N. and Matthews B.F. (1996). Identification of molecular markers in soybean comparing RFLP, RAPD, and AFLP DNA mapping techniques. Plant Mol. Biol. Report 14: 156–169 .
Nei M. and Li W.-H. (1979). Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 76: 5269–5273 .
Pandey R.M. (1999). Genet. Resour. Crop Evol. 46: 219–224 .
Seman K., Bjornstad A. and Stedje B. (2003). Genetic diversity and differentiation in Ethiopian populations of Phytolacca dodecandra as revealed by AFLP and RAPD analyses. Genet. Resour. Crop Evol. 50: 649–661 .
Swofford D.L. (2001). PAUP*. Phylogenetic Analysis Using Parsimony (and Other Methods). Sinauer Associates, Sunderland, Massachusetts .
Transue D.K., Fairbanks D.J., Robison L.R. and Andersen W.R. (1994). Species identification of RAPD analysis of grain amaranth genetic resources. Crop Sci. 34: 1385–1389 .
Treuren R. (2001). Identification of intra-accession genetic diversity in selfing crops using AFLP markers: implications for collection management. Genet. Resour. Crop Evol. 48: 287–295 .
Magda A., Hoekstra R., Treuren R. and Hintum T.J.L. (2004). Genetic and economic aspects of marker-assisted reduction of redundancy from a wild potato germplasm collection. Genet. Resour. Crop Evol. 51: 277–290 .
Vos P., Hogers R., Bleeker M., Rijans M., Hornes M., Frijters A., Pot J., Peleman J., Kuiper M., Zabeau M. and Lee T. (1995). AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23: 4407–4414 .
Xu F. and Sun M. (2001). Comparative analysis of phylogenetic relationships of grain amaranths and their wild relatives (Amaranthus; Amaranthaceae) using internal transcribed spaceramplified fragment length polymorphismand double-primer fluorescent intersimple sequence repeat markers. Mol. Phylogenet. Evol. 21: 372–387 .
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Costea, M., Brenner, D.M., Tardif, F.J. et al. Delimitation of Amaranthus cruentus L. and Amaranthus caudatus L. using micromorphology and AFLP analysis: an application in germplasm identification. Genet Resour Crop Evol 53, 1625–1633 (2006). https://doi.org/10.1007/s10722-005-2288-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10722-005-2288-3