Abstract
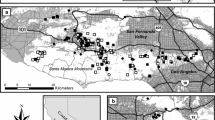
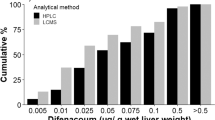
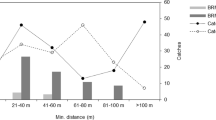
Second-generation anticoagulant rodenticides are widely reported to contaminate and poison nontarget wildlife, primarily predatory birds and mammals. Exposure pathways, however, have not been well defined. Here, we examined potential movement of rodenticides from deployment of bait to exposure of small mammals and other biota. At two adjacent working farms, we placed baits containing either brodifacoum or bromadiolone. We monitored movement of those compounds to the surrounding environment by collecting small mammals, birds, and invertebrates. Similar collections were made at a third agricultural setting without active bait deployment, but located among intensive livestock production and regular rodenticide use by farmers. Livers and whole invertebrate samples were analyzed for rodenticides using a sensitive LC-MSMS method. Norway rats (Rattus norvegicus) from both baited and non-baited farms had residues of brodifacoum or bromadiolone, implicating rats as an important exposure pathway to wildlife. Among 35 analyzed nontarget small mammals, a single vole had high hepatic residues (18.6 μ/g), providing some indication of a small mammal pathway. One song sparrow (Melospiza melodia) sample from a baited farm contained 0.073 μg/g of brodifacoum in liver, while 0.39 μg/g of diphacinone was measured in a pool of carrion beetles (Dermestes spp.) from the non-baited farm area, implicating avian and invertebrate components in exposure pathways. Regurgitated pellets of barn owl (Tyto alba) selected randomly from baited farms contained no detectable rodenticide residues, while 90 % of owl pellets collected from a variety of farms, and selected for the presence of rat fur, contained detectable anticoagulant residues. We recorded behavior of a captive sample of a representative songbird, the house sparrow (Passer domesticus); they readily entered bait stations and fed on (unloaded) bait.

Similar content being viewed by others
References
Albert, C. A., Mineau, P., Wilson, L. K., Trudeau, S., & Elliott, J. E. (2010). Rodenticide residues and autopsy data for three owl species from British Columbia, Canada. Archives Environmental Contamination and Toxicology, 58, 451–459.
Brakes, C. R., & Smith, C. H. (2005). Exposure of non-target small mammals to rodenticides: short-term effects, recovery and implications for secondary poisoning. Journal of Applied Ecology, 42, 118–128.
Buckle, A. P., Prescott, C. V., & Ward, K. J. (1994). Resistance to the first and second generation anticoagulant rodenticides—a new perspective. In W. S. Halverson & R. E. Marsh (Eds.), Proceedings of the Sixteenth Vertebrate Pest Conference (pp. 138–144). Lincoln: University of Nebraska–Lincoln.
Christensen, T. K., Lassen, P., & Elmeros, M. (2012). High exposure rates of anticoagulant rodenticides in predatory bird species in intensively managed landscapes in Denmark. Archives of Environmental Contamination and Toxicology, 63, 437–444.
Clare, J., Brantingham, P., & Brantingham, P. (2010). Problem-oriented policing approaches to outdoor cannabis growing (Public Safety Canada. Report No. 005 ISBN No.: 978-1-100-19935-1). ON: Ottawa.
Cox, P. R., & Smith, R. H. (1990). Rodenticide ecotoxicology: assessing non-target population effects. Functional Ecology, 4, 315–320.
Cox, P. R., & Smith, R. H. (1992). Rodenticide ecotoxicology: pre-lethal effects of anticoagulants on rat behaviour. In J. E. Borrecco & R. E. Marsh (Eds.), Proceedings of the fifteenth vertebrate pest conference (pp. 165–170). Davis: University of California.
Eason, C. T., Murphy, E., Wright, G. R., & Spurr, E. B. (2002). Assessment of risks of brodifacoum to non-target birds and mammals in New Zealand. Ecotoxicology, 11, 35–48.
Gabriel, M. W., Woods, L. W., Poppenga, R., Sweitzer, R. A., Thompson, C., Matthews, S. M., Higley, J. M., et al. (2012). Anticoagulant rodenticides on our public and community lands: spatial distribution of exposure and poisoning of a rare forest carnivore. PLoS One, 7, e40163.
Hegdal, P. L., & Colvin, B. A. (1988). Potential hazard to eastern screech-owls and other raptors of brodifacoum bait used for vole control in orchards. Environmental Toxicology and Chemistry, 7, 245–260.
Himsworth, C. G., Feng, A. Y., Parsons, K., Kerr, T., & Patrick, D. M. (2013). Using experiential knowledge to understand urban rat ecology: a survey of Canadian pest control professionals. Urban Ecosystems, 16, 341–350.
Hindmarch, S., Krebs, E. A., Elliott, J. E., & Green, D. J. (2012). Do landscape features predict the presence of barn owls in a rapidly changing agricultural landscape? Landscape and Urban Planning, 107, 255–262.
Hoare, J. M., & Kelly, K. M. (2006). The impact of brodifacoum on non-target wildlife: gaps in knowledge. New Zealand Journal of Ecology, 30, 157–167.
Howald, G. R. (1997). The risk of non-target species poisoning from brodifacoum used to eradicate rats from Langara Island, British Columbia, Canada (PhD dissertation), The University of British Columbia. BC: Vancouver.
Howald, G. R., Mineau, P., Elliott, J. E., & Cheng, K. M. (1999). Brodifacoum poisoning of avian scavengers during rat control on a seabird colony. Ecotoxicology, 8, 431–447.
Lambert, O., Pouliquen, H., Larhantec, M., Thorin, C., & L'Hostis, M. (2007). Exposure of raptors and waterbirds to anticoagulant rodenticides (difenacoum, bromadiolone, coumatetralyl, coumafen, brodifacoum): epidemiological survey in Loire Atlantique (France). Bulletin of Environmental Contamination and Toxicology, 79, 91–94.
Lima, L. L., & Salmon, T. P. (2010). Assessing some potential environmental impacts from agricultural anticoagulant uses. In R. M. Timm & K. A. Fagerstone (Eds.), Proceedings of the 24th Vertebrate Pest Conference (pp. 199–203). Davis: University of California Davis.
Mendenhall, V. M., & Pank, L. F. (1980). Secondary poisoning of owls by anticoagulant rodenticides. Wildlife Society Bulletin, 8, 311–315.
Merson, M. H., Byers, R. E., & Kaukeinen, D. E. (1984). Residues of the rodenticide brodifacoum in voles and raptors after orchard treatment. Journal of Wildlife Management, 48, 212–216.
Murray, M. (2011). Anticoagulant rodenticide exposure and toxicosis in four species of birds of prey presented to a wildlife clinic in Massachusetts, 2006–2010. Journal of Zoo and Wildlife Medicine, 42, 88–97.
Newton, I., Wyllie, I., & Freestone, P. (1990). Rodenticides in British barn owls. Environmental Pollution, 68, 101–117.
Newton, I., Wyllie, I., Gray, A., & Eadsforth, C. V. (1994). The toxicity of the rodenticide flocoumafen to barn owls and its elimination via pellets. Pesticide Science, 41, 187–193.
Newton, I., Afsarm A., Dale, L,, Finnie, J., Shore, R. F., Wright, J., Wyatt, C., Wyllie, I. (2000). Wildlife and Pollution. 1998/99 Annual Report, JNCC Report, No.305.
Newton, I., Shore, R. F., Wyllie, I., Birks, J. D. S., & Dale, L. (1999). Empirical evidence of side-effects of rodenticides on some predatory birds and mammals. In H. J. Pelz, D. P. Cowan, & C. J. Feare (Eds.), Advances in Vertebrate Pest Management (pp. 347–367). Furth: Filander.
Nimish, B. V., Hulse, C. S., Meteyer, C. U., & Rice, C. P. (2013). Evidence of songbird intoxication from Rozol® application at a black-tailed prairie dog colony. Journal of Fish and Wildlife Management, 4, 97–103.
Parmar, G., Bratt, H., Moore, R., & Batten, P. L. (1987). Evidence for a common binding-site in vivo for the retention of anticoagulants in rat liver. Human Toxicology, 6, 431–432.
Pimentel, D., Zuniga, R., & Morrison, D. (2005). Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecological Economics, 52, 273–288.
PMRA. 2006. Re-evaluation Decision Document. Brodifacoum, Bromadiolone, Chlorophacinone, Diphacinone and Warfarin. RRD2006-11. Pest Management Regulatory Agency, Health Canada, Ottawa, Ontario, Canada.
PMRA. 2010. Proposed Risk Mitigation Measures for Eight Rodenticides. REV2010-17. Pest Management Regulatory Agency, Health Canada, Ottawa, Ontario, Canada.
Quy, R. J., Cowan, D. P., Prescott, C. V., Gill, J. E., Kerins, G. M., Dunsford, G., Jones, A., & Macnicoll, A. D. (1995). Control of a population of Norway rats resistant to anticoagulant rodenticides. Pesticide Science, 45, 247–256.
Rattner, B. A., Horak, K. E., Warner, S. E., Day, D. D., Meteyer, C. U., Volker, S. F., Eisemann, J. D., & Johnston, J. J. (2011). Acute toxicity, histopathology, and coagulopathy in American kestrels (Falco sparverius) following administration of the rodenticide diphacinone. Environmental Toxicology and Chemistry, 30, 1213–1222.
Rattner, B. A., Horak, K. E., Lazarus, R. S., Eisenreich, K. M., Meteyer, C. U., Volker, S. F., Campton, C. M., Eisemann, J. D., & Johnston, J. J. (2012). Assessment of toxicity and potential risk of the anticoagulant rodenticide diphacinone using Eastern screech-owls (Megascops asio). Ecotoxicology, 21, 1–15.
Riley, S. P. D., Bromley, C., Poppenga, R. H., Uzai, F. A., Whited, L., & Sauvajot, R. M. (2007). Anticoagulant exposure and notoedric mange in bobcats and mountain lions in urban southern California. Journal of Wildlife Management, 7, 1874–1884.
Shore, R. F., Birks, J. D. S., Freestone, P., & Kitchener, A. C. (1996). Second-generation rodenticides and polecats (Mustela putorius) in Britain. Environmental Pollution, 91, 279–282.
Stone, W. B., Okoniewski, J. C., & Stedelin, J. R. (1999). Poisoning of wildlife with anticoagulant rodenticides in New York. Journal of Wildlife Disease, 35, 187–193.
Stone, W. B., Okoniewski, J. C., & Stedelin, J. R. (2003). Anticoagulant Rodenticides and Raptors: Recent Findings from New York, 1998–2001. Bulletin of Environmental Contamination and Toxicology, 70, 34–40.
Thijssen, H. H. W. (1995). Warfarin-based rodenticides: mode of action and mechanism of resistance. Pesticide Science, 43, 73–78.
Thomas, P. H., Mineau, P., Shore, R. F., Champoux, L., Martin, P. A., Wilson, L. K., Fitzgerald, G., & Elliott, J. E. (2011). Second generation anticoagulant rodenticides in predatory birds: probabilistic characterization of toxic liver concentrations and implications for predatory bird populations in Canada. Environment International, 37, 914–920.
Tobin, M. E., Sugihara, R. T., Koehler, A. E., & Ueunten, G. R. (1996). Seasonal activity and movements of Rattus rattus (Rodentia, Muridae) in a Hawaiian macadamia orchard. Mammalia, 60, 3–13.
US EPA. (2004). Potential Risks of Nine Rodenticides to Birds and Nontarget Mammals: A Comparative Approach. Environmental Protection Agency, Office of Pesticides Programs, Environmental Fate and Effects Division, Washington, DC, USA
US EPA. (2011). Risks of Non-Compliant Rodenticides to Non-target Wildlife. Background Paper for Science Advisory Panel on Notice of Intent to Cancel Non-RMD compliant Rodenticide Products. Environmental Protection Agencey, Office of Chemical Safety and Pollution Prevention. Washington, DC, USA
Walker, L. A., Turk, A., Long, S. M., Wienburg, C. L., Best, J., & Shore, R. F. (2008). Second generation anticoagulant rodenticides in tawny owls (Strix aluco) from Great Britain. Science of the Total Environment, 392, 93–98.
Watt, B. E., Proudfoot, A. T., Bradberry, S. M., & Vale, J. A. (2005). Anticoagulant rodenticides. Toxicological Reviews, 24, 259–269.
Acknowledgments
We thank the many cooperating farmers, particularly those at the intensive study sites in Delta, for making this work possible. Sandi Lee is thanked for her assistance with field studies. We thank the staff at the National Wildlife Research Center staff for specimen archiving and rodenticide residue analysis. Funding was primarily from the Pesticide Science Fund of Environment Canada.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elliott, J.E., Hindmarch, S., Albert, C.A. et al. Exposure pathways of anticoagulant rodenticides to nontarget wildlife. Environ Monit Assess 186, 895–906 (2014). https://doi.org/10.1007/s10661-013-3422-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-013-3422-x