Abstract
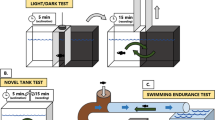
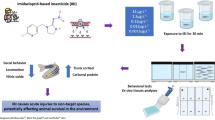
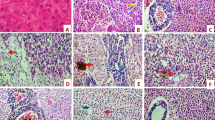
Ivermectin (IVM) is a broad acting antihelmintic used in various veterinary pharmaceuticals. It has been shown that IVM enters the aquatic compartment and adversely affects organisms including fish. This study is based on the hypothesis that long term exposure to IVM affects fish and thus, the main objective was to assess the chronic effects of 0.25 and 25 µg IVM/L to zebrafish using multiple endpoints representative of several levels of biological organization: weight, behaviour (swimming and feeding) and subcellular markers including biomarkers for oestrogenicity (vitellogenin-VTG), oxidative stress (catalase-CAT and glutathione-S-transferase-GST) and neurotransmission (cholinesterase-ChE). Concentrations as low as 0.25 µg IVM/L disrupted the swimming behaviour, causing fish to spend more time at the bottom of aquaria. Such reduction of the swimming performance affected the feeding ability which is likely responsible for the weight loss. The effects on weight were gender differentiated, being more pronounced in males (0.25 µg IVM/L) than in females (25 µg IVM/L). Fish exposed to 25 µg/L exhibited darker coloration and mild curvature of the spine. No effects on VTG and AChE were observed, but a reduction on CAT and GST levels was observed in fish exposed to 25 µg IVM/L, although these alterations probably only reflect the general condition of the fish which was significantly compromised at this concentration. Despite that predicted environmental concentrations of IVM are below 0.25 µg/L, the behavioural effects may be translated into important ecological impacts, e.g. at predator–prey interactions where fish competitive advantage can be decreased. Future work should address the link between behaviour disruption and population fitness. The current study was based on a one experiment and multiple endpoint (anchored) approach, allowing the results to be integrated and linked towards a mechanistic understanding.




Similar content being viewed by others
References
Boonstra H, Reichman EP, van den Brink PJ (2011) Effects of the veterinary pharmaceutical ivermectin in indoor aquatic microcosms. Arch Environ Contam Toxicol 60:77–89
Boxall ABA, Fogg LA, Blackwell PA, Kay P, Pemberton EJ (2002) Review of veterinary medicines in the environment. R&D Technical Report P6-012/8/TR, Cranfield Centre for EcoChemistry. Environment Agency, Bristol
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brinke M, Hoess S, Fink G, Ternes TA, Heininger P, Traunspurger W (2010) Assessing effects of the pharmaceutical ivermectin on meiobenthic communities using freshwater microcosms. Aquat Toxicol 99:126–137
Cannavan A, Coyne R, Kennedy DG, Smith P (2000) Concentration of 22,23-dihydroavermectin B-1a detected in the sediments at an Atlantic salmon farm using orally administered ivermectin to control sea-lice infestation. Aquaculture 182:229–240
Carlsson G, Patring J, Kreuger J, Norrgren L, Oskarsson A (2013) Toxicity of 15 veterinary pharmaceuticals in zebrafish (Danio rerio) embryos. Aquat Toxicology 126:30–41
Clairborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods in oxygen radical research. Boca Raton, FL, pp 283–284
Davies IM, Rodger GK (2000) A review of the use of ivermectin as a treatment for sea lice Lepeophtheirus salmonis (Krøyer) and Caligus elongatus Nordmann infestation in farmed Atlantic salmon (Salmo salar L.). Aquac Res 31(11):869–883
Duce IR, Scott RH (1985) Actions of dihydroavermectin B on insect muscle. Br J Pharmacol 85:395–401
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
EMEA, European Medicines Agency (2008) Revised guideline on environmental impact assessment for veterinary medicinal products in support of the VICH guidelines GL6 and GL38. London: Committee for Medicinal Products for Veterinary Use (CVMP), EMEA. EMEA/CVMP/ERA/418282/2005-Rev.1
Frasco MF, Guilhermino L (2003) Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata. Fish Physiol Biochem 26(2):149–156
Garric J, Vollat B, Duis K, Pery A, Junker T, Ramil M, Fink G, Ternes TA (2007) Effects of the parasiticide ivermectin on the cladoceran Daphnia magna and the green alga Pseudokirchneriella subcapitata. Chemosphere 69:903–910
Habig WH, Jakoby WB (1981) Assays for differentiation of glutathione S-transferases. Methods Enzymol 77:398–405
Halley BA, Jacob TA, Lu AYH (1989) The environmental impact of the use of ivermectin: environmental effects and fate. Chemosphere 18:1543–1563
Henry TB, McPherson JT, Rogers ED, Heah TP, Hawkins SA, Layton AC, Sayler GS (2009) Changes in the relative expression pattern of multiple vitellogenin genes in adult male and larval zebrafish exposed to exogenous estrogens. Comp Biochem Physiol A 154:119–126
Howcroft CF et al (2009) Effects of natural and chemical stressors on Enchytraeus albidus: can oxidative stress parameters be used as fast screening tools for the assessment of different stress impacts in soils? Environ Int 35:318–324
Hoy T, Horsberg TE, Nafstad I (1990) The disposition of ivermectin in atlantic salmon (Salmo salar). Pharmacol Toxicol 67(4):307–312
Jemec A, Drobne D, Tisler T, Sepcic K (2010) Biochemical biomarkers in environmental studies-lessons learnt from enzymes catalase, glutathione S-transferase and cholinesterase in two crustacean species. Environ Sci Pollut Res 17:571–581
Jin YX, Chen RJ, Sun LW, Qian HF, Liu WP, Fu ZW (2009) Induction of estrogen-responsive gene transcription in the embryo, larval, juvenile and adult life stages of zebrafish as biomarkers of short-term exposure to endocrine disrupting chemicals. Comp Biochem Physiol C 150:414–420
Katharios P, Pavlidis M, Iliopoulou-Georgudaki J (2004) Accumulation of ivermectin in the brain of sea bream, Sparus aurata after intraperitoneal administration. Environ Toxicol Pharmacol 17:9–12
Kennedy CJ, Tierney KB, Mittelstadt M (2014) Inhibition of P-glycoprotein in the blood-brain barrier alters avermectin neurotoxicity and swimming performance in rainbow trout. Aquat Toxicol 146:176–185
Kilmartin J, Cazabon D, Smith P (1996) Investigations of the toxicity of ivermectin for salmonids. Bull Eur Assoc Fish Pathol 17:58–61
Kools SAE, Moltmann JF, Knacker T (2008) Estimating the use of veterinary medicines in the European union. Regul Toxicol Pharm 50:59–65
Kruger K, Scholtz CH (1995) The effect of ivermectin on the development and reproduction of the dung-breeding fly Musca nevilli Kleynhans (Diptera, Muscidae). Agric Ecosyst Environ 53:13–18
Li M, You T-Z, Zhu W-J, Qu J-P, Liu C, Zhao B, Xu S-W, Li S (2013) Antioxidant response and histopathological changes in brain tissue of pigeon exposed to avermectin. Ecotoxicology 22:1241–1254
Liebig M et al (2010) Environmental risk assessment of ivermectin: a case study. Integr Environ Assess Manage 6(Suppl):567–587
Lunke MD, Kaufman WR (1992) Effects of the avermectin analogue MK-243 on vitellogenesis and reproduction in the ixodid tick, Amblyomma hebraeum. Exp Appl Acarol 13:249–259
Metcalfe CD, Alder AC, Halling-Sorenson B, Krogh K, Fenner K (2008) Exposure assessment methods for veterinary and human-use medicines in the environment: PEC vs. MEC comparisons. In: Kummerer K (ed) Pharmaceuticals in the environment—sources, fate, effects and risks, 3rd edn. Springer, Heidelberg, pp 147–174
Novelli A, Vieira BH, Cordeiro D, Cappelini LTD, Vieira EM, Espíndola ELG (2012) Lethal effects of abamectin on the aquatic organisms Daphnia similis, Chironomus xanthus and Danio rerio. Chemosphere 86(1):36–40
OECD, Organization for Economic Cooperation and Development (1984) OECD Guidelines for the testing of chemicals, Test No 204: fish, prolonged toxicity test: 14-day Study
OECD, Organization for Economic Cooperation and Development (1992) OECD Guidelines for the testing of chemicals test No 203: Fish acute toxicity test
OECD, Organization for Economic Cooperation and Development (2000) OECD Guidelines for the testing of chemicals, test No 215: fish, Juvenile Growth Test
OECD, Organization for Economic Cooperation and Development (2008) Revised Draft OECD guidelines for the testing of chemicals—the fish screening assay for endocrine active substances
Omura S (2002) Mode of action of avermectin. In: Omura S (ed) Macrolide antibiotics: chemistry, biology and practice. Academic Press, New York, pp 571–576
Pait AS, Nelson JO (2009) A survey of indicators for reproductive endocrine disruption in Fundulus heteroclitus (killifish) at selected sites in the Chesapeake Bay. Mar Environ Res 68:170–177
Prasse C, Loffler D, Ternes TA (2009) Environmental fate of the anthelmintic ivermectin in an aerobic sediment/water system. Chemosphere 77:1321–1325
Rico A, Van der Brink P (2014) Probabilistic risk assessment of veterinary medicines applied to four major aquaculture species produced in Asia. Sci Total Environ 468–469:630–641
Sakin F, Yonar SM, Yonar ME, Saglam N (2012) Changes in selected immunological parameters and oxidative stress responses in different organs of Oncorhynchus mykiss exposed to ivermectin. Rev Chim 63:989–995
Sanderson H, Laird B, Pope L, Brain R, Wilson C, Johnson D, Bryning G, Peregrine AS, Boxall A, Solomon K (2007) Assessment of the environmental fate and effects of ivermectin in aquatic mesocosms. Aquat Toxicol 85:229–240
Stevens J, Breckenridge CB (2001) The avermectins: insecticidal and antiparasitic agentes, Chapter 56. In: Krieger R (ed) Handbook of pesticide toxicology: principles and agents, vol 1. Academic Press, New York
Ucan-Marin F, Ernst W, O’Dor RK, Sherry J (2012) Effects of food borne ivermectin on juvenile Atlantic salmon (Salmo salar L.): survival, growth, behavior, and physiology. Aquaculture 334:169–175
Varo I, Rigos G, Navarro JC, del Ramo J, Calduch-Giner J, Hernandez A, Pertusa J, Torreblanca A (2010) Effect of ivermectin on the liver of gilthead sea bream Sparus aurata: a proteomic approach. Chemosphere 80:570–577
VICH (2000) Environmental impact assessment for veterinary medicinal products—phase I. International cooperation on harmonisation of technical requirements for registration of veterinary products
VICH (2004) Environmental impact assessment for veterinary medicinal products- phase II. International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary products
Wolstenholme AJ, Rogers AT (2005) Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology 131:S85–S95
Acknowledgments
This study was funded by FEDER through COMPETE and Programa Operacional Factores de Competitividade and by National funding through FCT-Fundação para a Ciência e Tecnologia, within FUBIA FCOMP-01-0124-FEDER-008651 (Ref. PTDC/AAC-CLI/103719/2008) and Climatox FCOMP-01-0124-FEDER-027795 (Ref. PTDC/AAG-GLO/4059/2012) and a Post-Doc grant to Inês Domingues (SFRH/BPD/90521/2012). The authors also acknowledge the Conselho Nacional de Desenvolvimento Cientifico e Tecnologico, CNPq, (Brazilian National Science Foundation), through the “Ciência sem Fronteiras” program. The authors acknowledge the help provided by Pedro Leal in the laboratorial experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The procedures described in the present paper respect national and international safety regulations and ethical principles for animal welfare.
Rights and permissions
About this article
Cite this article
Domingues, I., Oliveira, R., Soares, A.M.V.M. et al. Effects of ivermectin on Danio rerio: a multiple endpoint approach: behaviour, weight and subcellular markers. Ecotoxicology 25, 491–499 (2016). https://doi.org/10.1007/s10646-015-1607-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-015-1607-5