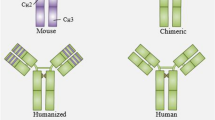
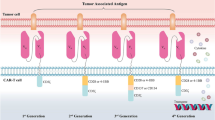
Summary
Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) contain 12 family members(CEACAM1、CEACAM3、CEACAM4、CEACAM5、CEACAM6、CEACAM7、CEACAM8、CEACAM16、CEACAM18、CEACAM19、CEACAM20 and CEACAM21)and are expressed diversely in different normal and tumor tissues. CEA (CEACAM5) has been used as a tumor biomarker since 1965. Here we review the latest research and development of the structures, expression, and function of CEACAMs in normal and tumor tissues, and their application in the tumor diagnosis, prognosis, and treatment. We focus on recent clinical studies of CEA targeted cancer immunotherapies, including bispecific antibody (BsAb) for radio-immuno-therapy and imaging, bispecific T cell engager (BiTE) and chimeric antigen receptor T cells (CAR-T). We summarize the promising clinical relevance and challenges of these approaches and give perspective view for future research. This review has important implications in understanding the diversified biology of CEACAMs in normal and tumor tissues, and their new role in tumor immunotherapy.

Similar content being viewed by others
Change history
22 June 2020
Correction is needed to the original version of this article.
Abbreviations
- BiTE:
-
Bispecific T Cell Engager.
- BsAb:
-
Bispecific Antibody.
- CEA:
-
Carcinoembryonic Antigen.
- CEACAM:
-
Carcinoembryonic Antigen-Related Cell Adhesion Molecule.
- CAR-T:
-
Chimeric Antigen Receptor T Cell.
- HSG:
-
Histamine-succinylglycine.
- Ig:
-
Immunoglobulin.
- TAA:
-
Tumor-associated antigens.
- HAI:
-
Hepatic Intraarterial Infusion.
- SIRT:
-
Selective Internal Radiation Therapy.
References
Taheri M et al (2000) Self recognition in the Ig superfamily. Identification of precise subdomains in carcinoembryonic antigen required for intercellular adhesion. J Biol Chem 275(35):26935–26943
Gold P, Freedman SO (1965) Specific carcinoembryonic antigens of the human digestive system. J Exp Med 122(3):467–481
Beauchemin N, Arabzadeh A (2013) Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev 32(3–4):643–671
Kuespert K, Pils S, Hauck CR (2006) CEACAMs: their role in physiology and pathophysiology. Curr Opin Cell Biol 18(5):565–571
Horst AK, Wagener C (2004) CEA-Related CAMs. Handb Exp Pharmacol 165:283–341
Gray-Owen SD, Blumberg RS (2006) CEACAM1: contact-dependent control of immunity. Nat Rev Immunol 6(6):433–446
Sedykh SE et al (2018) Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther 12:195–208
Baeuerle PA, Reinhardt C (2009) Bispecific T-cell engaging antibodies for cancer therapy. Cancer Res 69(12):4941–4944
Frankel SR, Baeuerle PA (2013) Targeting T cells to tumor cells using bispecific antibodies. Curr Opin Chem Biol 17(3):385–392
Wolf E et al (2005) BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov Today 10(18):1237–1244
Kochenderfer JN et al (2015) Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 33(6):540–549
Offner S et al (2006) Induction of regular cytolytic T cell synapses by bispecific single-chain antibody constructs on MHC class I-negative tumor cells. Mol Immunol 43(6):763–771
Naghibalhossaini F, Stanners CP (2004) Minimal mutations are required to effect a radical change in function in CEA family members of the Ig superfamily. J Cell Sci 117(Pt 5):761–769
Dery KJ et al (2011) Mechanistic control of carcinoembryonic antigen-related cell adhesion molecule-1 (CEACAM1) splice isoforms by the heterogeneous nuclear ribonuclear proteins hnRNP L, hnRNP A1, and hnRNP M. J Biol Chem 286(18):16039–16051
Wang L et al (2000) C-CAM1, a candidate tumor suppressor gene, is abnormally expressed in primary lung cancers. Clin Cancer Res 6(8):2988–2993
Markel G et al (2004) Biological function of the soluble CEACAM1 protein and implications in TAP2-deficient patients. Eur J Immunol 34(8):2138–2148
Simeone DM et al (2007) CEACAM1, a novel serum biomarker for pancreatic cancer. Pancreas 34(4):436–443
Tilki D et al (2010) CEACAM1: a novel urinary marker for bladder cancer detection. Eur Urol 57(4):648–654
Dankner M et al (2017) CEACAM1 as a multi-purpose target for cancer immunotherapy. Oncoimmunology 6(7):e1328336
Frangsmyr L et al (1995) Cell- and region-specific expression of biliary glycoprotein and its messenger RNA in normal human colonic mucosa. Cancer Res 55(14):2963–2967
Nolan KF et al (1999) Bypassing immunization: optimized design of “designer T cells” against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA. Clin Cancer Res 5(12):3928–3941
Hammarstrom S (1999) The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol 9(2):67–81
Nap M et al (1988) Immunohistochemistry of carcino-embryonic antigen in the embryo, fetus and adult. Tumour Biol 9(2–3):145–153
Yan Z et al (1997) Oncogenic c-Ki-ras but not oncogenic c-Ha-ras up-regulates CEA expression and disrupts basolateral polarity in colon epithelial cells. J Biol Chem 272(44):27902–27907
Scholzel S et al (2000) Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol 156(2):595–605
Gambichler T et al (2009) Protein expression of carcinoembryonic antigen cell adhesion molecules in benign and malignant melanocytic skin lesions. Am J Clin Pathol 131(6):782–787
Thies A et al (2007) Glycoconjugate profiling of primary melanoma and its sentinel node and distant metastases: implications for diagnosis and pathophysiology of metastases. Cancer Lett 248(1):68–80
Neumaier M et al (1993) Biliary glycoprotein, a potential human cell adhesion molecule, is down-regulated in colorectal carcinomas. Proc Natl Acad Sci U S A 90(22):10744–10748
Kunath T et al (1995) Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene 11(11):2375–2382
Nollau P et al (1997) Expression of CD66a (human C-CAM) and other members of the carcinoembryonic antigen gene family of adhesion molecules in human colorectal adenomas. Cancer Res 57(12):2354–2357
Turbide C et al (1997) Optimal ratios of biliary glycoprotein isoforms required for inhibition of colonic tumor cell growth. Cancer Res 57(13):2781–2788
Izzi L et al (1999) cis-Determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene 18(40):5563–5572
Yeatman TJ, Mao W, Karl RC (1997) Biliary glycoprotein is overexpressed in human colon cancer cells with high metastatic potential. J Gastrointest Surg 1(3):292–298
Kang WY et al (2007) The expression of CD66a and possible roles in colorectal adenoma and adenocarcinoma. Int J Colorectal Dis 22(8):869–874
Song JH et al (2011) Genetic alterations and expression pattern of CEACAM1 in colorectal adenomas and cancers. Pathol Oncol Res 17(1):67–74
Ou G et al (2009) Contribution of intestinal epithelial cells to innate immunity of the human gut–studies on polarized monolayers of colon carcinoma cells. Scand J Immunol 69(2):150–161
Arabzadeh A et al (2016) Carcinoembryonic Antigen Cell Adhesion Molecule 1 long isoform modulates malignancy of poorly differentiated colon cancer cells. Gut 65(5):821–829
Zhou MQ et al (2013) Clinical and experimental studies regarding the expression and diagnostic value of carcinoembryonic antigen-related cell adhesion molecule 1 in non-small-cell lung cancer. BMC Cancer 13:359
Sienel W et al (2003) Elevated expression of carcinoembryonic antigen-related cell adhesion molecule 1 promotes progression of non-small cell lung cancer. Clin Cancer Res 9(6):2260–2266
Nolen BM et al (2015) Urinary protein biomarkers in the early detection of lung cancer. Cancer Prev Res (Phila) 8(2):111–119
Gong DY et al (2011) [Diagnostic value of serum CEACAM1 in patients with pancreatic cancer]. Nan Fang Yi Ke Da Xue Xue Bao 31(1):164–166
Giulietti M et al (2016) Weighted gene co-expression network analysis reveals key genes involved in pancreatic ductal adenocarcinoma development. Cell Oncol (Dordr) 39(4):379–388
Gebauer F et al (2014) Carcinoembryonic antigen-related cell adhesion molecules (CEACAM) 1, 5 and 6 as biomarkers in pancreatic cancer. PLoS One 9(11):e113023
Oliveira-Ferrer L et al (2004) Dual role of carcinoembryonic antigen-related cell adhesion molecule 1 in angiogenesis and invasion of human urinary bladder cancer. Cancer Res 64(24):8932–8938
Kleinerman DI et al (1996) Suppression of human bladder cancer growth by increased expression of C-CAM1 gene in an orthotopic model. Cancer Res 56(15):3431–3435
Yang M et al (2015) T-cell immunoglobulin mucin-3 expression in bladder urothelial carcinoma: Clinicopathologic correlations and association with survival. J Surg Oncol 112(4):430–435
Fichera A, Michelassi F, Arenas RB (1998) Selective expression of carcinoembryonic antigen promoter in cancer cell lines: targeting strategy for gene therapy in colorectal cancer. Dis Colon Rectum 41(6):747–754
Tiernan JP et al (2013) Carcinoembryonic antigen is the preferred biomarker for in vivo colorectal cancer targeting. Br J Cancer 108(3):662–667
Hanenberg H et al (1994) Expression of the CEA gene family members NCA-50/90 and NCA-160 (CD66) in childhood acute lymphoblastic leukemias (ALLs) and in cell lines of B-cell origin. Leukemia 8(12):2127–2133
Jantscheff P et al (2003) Expression of CEACAM6 in resectable colorectal cancer: a factor of independent prognostic significance. J Clin Oncol 21(19):3638–3646
Kim KS et al (2013) Overexpression and clinical significance of carcinoembryonic antigen-related cell adhesion molecule 6 in colorectal cancer. Clin Chim Acta 415:12–19
Blumenthal RD et al (2007) Expression patterns of CEACAM5 and CEACAM6 in primary and metastatic cancers. BMC Cancer 7:2
Thompson J et al (1994) CGM2, a member of the carcinoembryonic antigen gene family is down-regulated in colorectal carcinomas. J Biol Chem 269(52):32924–32931
Zhou J et al (2011) Dynamic expression of CEACAM7 in precursor lesions of gastric carcinoma and its prognostic value in combination with CEA. World J Surg Oncol 9:172
Ellison LM et al (2017) Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in treatment of gastric cancer with peritoneal carcinomatosis. Chin J Cancer Res 29(1):86–92
Xin HW et al (2016) Liver label retaining cancer cells are relatively resistant to the reported anti-cancer stem cell drug metformin. J Cancer 7(9):1142–1151
Xin HW et al (2013) Wnt and the cancer niche: paracrine interactions with gastrointestinal cancer cells undergoing asymmetric cell division. J Cancer 4(6):447–457
Xin HW et al (2013) Label-retaining liver cancer cells are relatively resistant to sorafenib. Gut 62(12):1777–1786
Xin HW et al (2012) Tumor-initiating label-retaining cancer cells in human gastrointestinal cancers undergo asymmetric cell division. Stem Cells 30(4):591–598
Goel G, Sun W (2015) Novel approaches in the management of pancreatic ductal adenocarcinoma: potential promises for the future. J Hematol Oncol 8:44
Ma W et al (2016) Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol 9(1):47
Hirsch FR et al (2016) New and emerging targeted treatments in advanced non-small-cell lung cancer. Lancet 388(10048):1012–1024
Wang D et al (2018) CRISPR/Cas9 genome editing technology significantly accelerated herpes simplex virus research. Cancer Gene Ther 25(5–6):93–105
Wang YY et al (2019) Identification of putative UL54 (ICP27) transcription regulatory sequences binding to Oct-1, v-Myb, Pax-6 and hairy in herpes simplex viruses. J Cancer 10(2):430–440
Liu XQ et al (2018) Oncolytic herpes simplex virus tumor targeting and neutralization escape by engineering viral envelope glycoproteins. Drug Deliv 25(1):1950–1962
McCardle RJ et al (1966) Studies with iodine-131-labeled antibody to human fibrinogen for diagnosis and therapy of tumors. J Nucl Med 7(11):837–847
Wu ZJ et al (2018) Oncolytic viruses for tumor precision imaging and radiotherapy. Hum Gene Ther 29(2):204–222
Abdel Razek AAK (2018) Routine and advanced diffusion imaging modules of the salivary glands. Neuroimaging Clin N Am 28(2):245–254
Abdel Razek AAK et al (2019) Clinical applications of arterial spin labeling in brain tumors. J Comput Assist Tomogr 43(4):525–532
Razek AA, Nada N (2016) Correlation of choline/creatine and apparent diffusion coefficient values with the prognostic parameters of head and neck squamous cell carcinoma. NMR Biomed 29(4):483–489
Goldenberg DM et al (1978) Use of radiolabeled antibodies to carcinoembryonic antigen for the detection and localization of diverse cancers by external photoscanning. N Engl J Med 298(25):1384–1386
Dallas MR et al (2012) Divergent roles of CD44 and carcinoembryonic antigen in colon cancer metastasis. FASEB J 26(6):2648–2656
Francis RJ et al (2002) A phase I trial of antibody directed enzyme prodrug therapy (ADEPT) in patients with advanced colorectal carcinoma or other CEA producing tumours. Br J Cancer 87(6):600–607
Reck M, Heigener D, Reinmuth N (2016) Immunotherapy for small-cell lung cancer: emerging evidence. Future Oncol 12(7):931–943
Schoffelen R et al (2010) Pretargeted immuno-positron emission tomography imaging of carcinoembryonic antigen-expressing tumors with a bispecific antibody and a 68 Ga- and 18F-labeled hapten peptide in mice with human tumor xenografts. Mol Cancer Ther 9(4):1019–1027
Bodet-Milin C et al (2019) Clinical results in medullary thyroid carcinoma suggest high potential of pretargeted immuno-PET for tumor imaging and theranostic approaches. Front Med (Lausanne) 6:124
Sharkey RM et al (2010) Recombinant bispecific monoclonal antibodies prepared by the dock-and-lock strategy for pretargeted radioimmunotherapy. Semin Nucl Med 40(3):190–203
Bodet-Milin C et al (2016) Immuno-PET using anticarcinoembryonic antigen bispecific antibody and 68 Ga-labeled peptide in metastatic medullary thyroid carcinoma: clinical optimization of the pretargeting parameters in a first-in-human trial. J Nucl Med 57(10):1505–1511
Foubert F et al (2018) Sensitivity of pretargeted immunoPET using (68)Ga-peptide to detect colonic carcinoma liver metastases in a murine xenograft model: comparison with (18)FDG PET-CT. Oncotarget 9(44):27502–27513
Zhao Y et al (2020) Identification of anti-CD16a single domain antibodies and their application in bispecific antibodies. Cancer Biol Ther 21(1):72–80
Bodet-Milin C et al (2015) Pharmacokinetics and dosimetry studies for optimization of pretargeted radioimmunotherapy in CEA-expressing advanced lung cancer patients. Front Med (Lausanne) 2:84
Moek KL et al (2019) 89Zr-labeled bispecific T-cell engager AMG 211 PET shows AMG 211 accumulation in CD3-rich tissues and clear, heterogeneous tumor uptake. Clin Cancer Res
Pishvaian M et al (2016) Phase 1 dose escalation study of MEDI-565, a bispecific T-cell engager that targets human carcinoembryonic antigen, in patients with advanced gastrointestinal adenocarcinomas. Clin Colorectal Cancer 15(4):345–351
Brischwein K et al (2007) Strictly target cell-dependent activation of T cells by bispecific single-chain antibody constructs of the BiTE class. J Immunother 30(8):798–807
Hoffmann P et al (2005) Serial killing of tumor cells by cytotoxic T cells redirected with a CD19-/CD3-bispecific single-chain antibody construct. Int J Cancer 115(1):98–104
Molhoj M et al (2007) CD19-/CD3-bispecific antibody of the BiTE class is far superior to tandem diabody with respect to redirected tumor cell lysis. Mol Immunol 44(8):1935–1943
Amann M et al (2009) Antitumor activity of an EpCAM/CD3-bispecific BiTE antibody during long-term treatment of mice in the absence of T-cell anergy and sustained cytokine release. J Immunother 32(5):452–464
Oberst MD et al (2014) CEA/CD3 bispecific antibody MEDI-565/AMG 211 activation of T cells and subsequent killing of human tumors is independent of mutations commonly found in colorectal adenocarcinomas. MAbs 6(6):1571–1584
Pan H et al (2018) Site-specific PEGylation of an anti-CEA/CD3 bispecific antibody improves its antitumor efficacy. Int J Nanomedicine 13:3189–3201
Thistlethwaite FC et al (2017) The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer Immunol Immunother 66(11):1425–1436
Zhang C et al (2017) Phase I escalating-dose trial of CAR-T therapy targeting CEA(+) metastatic colorectal cancers. Mol Ther 25(5):1248–1258
Katz SC et al (2015) Phase I hepatic immunotherapy for metastases study of intra-arterial chimeric antigen receptor-modified T-cell therapy for CEA + liver metastases. Clin Cancer Res 21(14):3149–3159
Hombach AA, Rappl G, Abken H (2019) Blocking CD30 on T cells by a dual specific CAR for CD30 and colon cancer antigens improves the CAR T cell response against CD30(-) Tumors. Mol Ther 27(10):1825–1835
Katz SC et al (2019) HITM-SIR: phase Ib trial of intraarterial chimeric antigen receptor T-cell therapy and selective internal radiation therapy for CEA(+) liver metastases. Cancer Gene Ther
Funding
This work was mainly supported by National Natural Science Foundation of China (81872412 to XHW, 81602303 to XY, 31700736 to WXW). We thank Hubei Province Natural Science Foundation of China (2016CFB180 to WXW), Foundation of Health and Family Planning Commission of Hubei Province (WJ2016-Y-02 To MZ, WJ2016Y07 To WXW), Hubei Province Scientific and Technological Research Project (Q20171306 to XWW), Jingzhou Science and Technology Development Planning Project (JZKJ15063 to WXW), and Guangzhou Key Medical Discipline Construction Project (CSZ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest in this work.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Han, ZW., Lyv, ZW., Cui, B. et al. The old CEACAMs find their new role in tumor immunotherapy. Invest New Drugs 38, 1888–1898 (2020). https://doi.org/10.1007/s10637-020-00955-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-020-00955-w